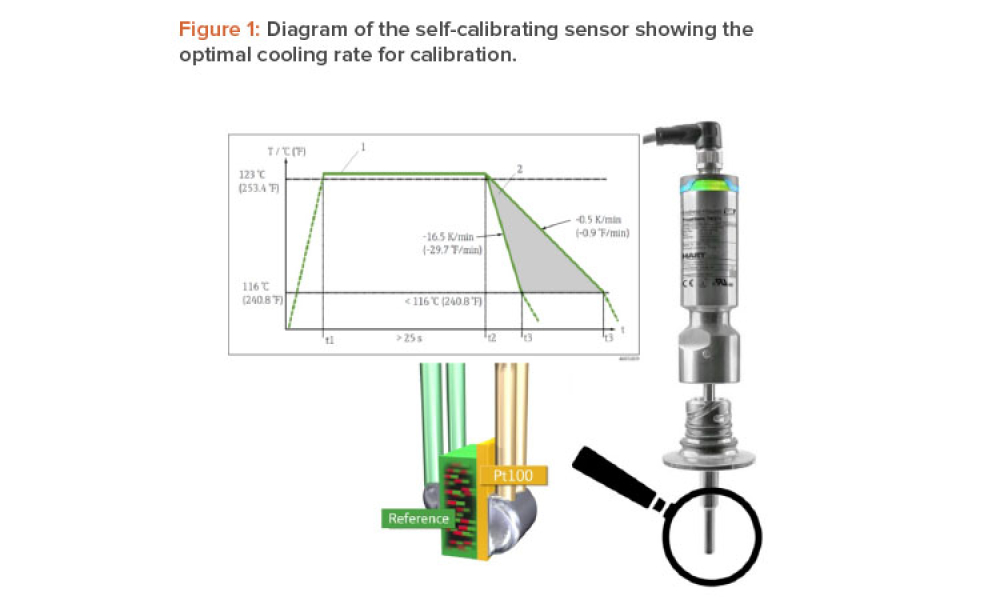
A temperature sensor in a medical autoclave is typically calibrated once a year. If the sensor proves to be inaccurate, all batches produced since the last calibration must be evaluated. Endress+Hauser has developed a self-calibrating sensor that automatically verifies its accuracy during each sterilization batch. This article describes a case study at the Merck Healthcare KGaA sterile...

Downloads
Technology Trends: The Transition to Digitalization
Cover: In the pharmaceutical industry, digitalization involves developing and implementing digital technologies at all levels of pharmaceutical operations. The aim is to transform the industry by capturing, analyzing, and using vast amounts of data collected from a wide range of sources to support research and development, clinical development, drug manufacturing, supply chain management, patient engagement, quality assurance and quality control, product safety monitoring, and other objectives.
Validation of Clinical Trial–Related Systems in Smaller Enterprises
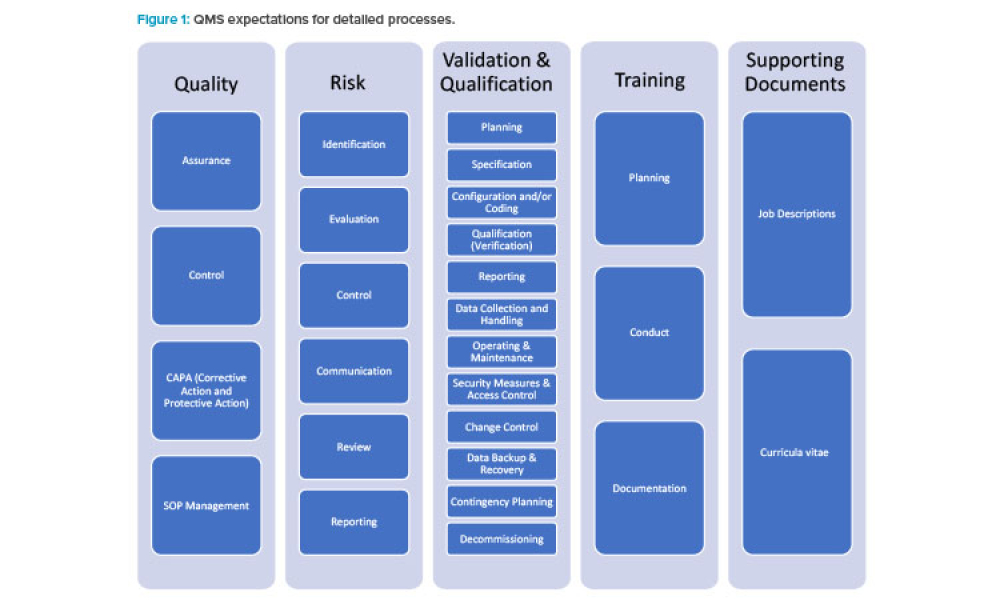
Feature: Existing risk-based approaches to computerized system compliance and validation as outlined in GAMP® 5 are applicable to a variety of life sciences organizations supporting or performing GxP-relevant activities. However, specific guidance on how to implement all the necessary measures and what to prioritize in small and medium sized enterprises is scarce.
ISPE France Affiliate: Long-Time Affiliate Continues to Shine
Feature: The ISPE France Affiliate is fortunate in many ways. The pharmaceutical industry in France is world class, employing close to 100,000 people and generating €55.9 billion in annual revenue. The Affiliate’s membership runs the gamut from students and Young Professionals to industry veterans with expertise in research and development, engineering, manufacturing, and regulatory guidelines.
Special Section: 2020 ISPE Annual Meeting & Expo
- 2020 ISPE Annual Meeting & Expo: Driving the Future of Pharma
- FOYA Category Winners & Honorable Mentions for 2020: Examples of Excellence
Quality and Regulatory Solutions for PAT in Continuous Manufacturing
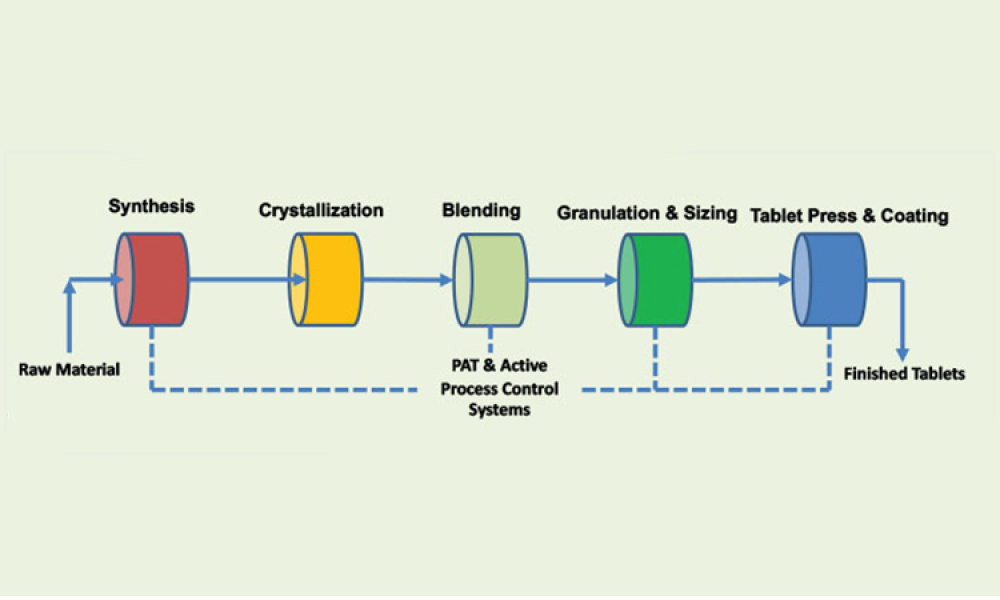
Technical: This article discusses quality and regulatory hurdles in the life cycle of a process analytical technology application— including model life-cycle management—in combination with continuous manufacturing for small and large molecules, with the goal of proposing strategies to resolve each challenge.
In This Issue
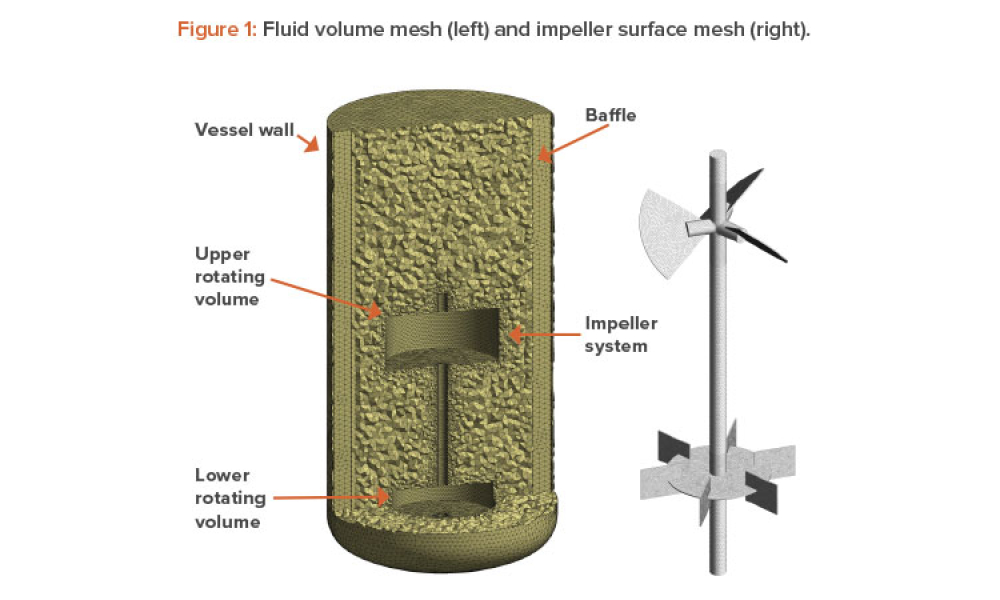
Computational fluid dynamics (CFD) can reduce or eliminate the need to perform bioreactor scale-up studies because full-scale manufacturing bioreactors can be simulated to predict performance. This article discusses the use of computational fluid dynamics for that purpose, to predict the performance of a manufacturing-scale bioreactor under various operating conditions.
The 2020 ISPE Annual Meeting & Expo will be ISPE’s first completely virtual Annual Meeting. As always, there will be great learning and networking opportunities—in fact, the digital format offers greater flexibility for attendees.
Process analytical technology (PAT) is perceived as the main enabler for a robust control strategy with continuous manufacturing (CM) because process analytical technology can aid in implementing continuous manufacturing throughout the entire life cycle. This article discusses quality and regulatory hurdles in the life cycle of a process analytical technology application—including model...
In honor of the 40th birthday of ISPE and Pharmaceutical Engineering®, we looked back to 2010 to see what technology-focused articles were published in the magazine. Here are just a few:
Existing risk-based approaches to computerized system compliance and validation as outlined in GAMP® 5
The ISPE France Affiliate is fortunate in many ways. The pharmaceutical industry in France is world class, employing close to 100,000 people and generating €55.9 billion in annual revenue.
Each year, ISPE celebrates innovations and advances in pharmaceutical manufacturing technology with its Facility of the Year Awards (FOYA) program. This year, we added a new category, Social Impact, to recognize companies that developed new standards and practices to prevent drug shortages and increase patients’ access to medicine, designed new tools or techniques that reduced the cost of drug...
In March 2020, the ISPE Eurasian Economic Union (EAEU) Affiliate was officially established. This event was a significant step along the journey to integrate members from Eurasian Economic Union nations into the international pharmaceutical community, as well as another sign of progress in the development of a harmonized scientific,...
ISPE has published a new Good Practice Guide: Critical Utilities GMP Compliance—How to Be Compliant and Ready to Prove It. Written and reviewed by...
How many times have you heard phrases like these over the last several months: unprecedented marketplace disruptions, staggering economic conditions, or maybe insurmountable business challenges? If you’re like me, probably more than you can count.
Because of the global pandemic, we have experienced unexpected joys, learned new skills, adjusted to long days of video conferencing, and dealt with drops in income and potential job losses. At the same time, we have also experienced an increased sense of urgency, collaboration, and pride because we are a part of the industry that has been tapped to heal all our nations from this unexpected...
As many of you read this, I am sure your social and work life have been turned upside down, flipped around, and now are possibly settling into “the new normal.” I personally cringed when I wrote that—I was so tired of hearing that phrase about three weeks into the pandemic.
In the pharmaceutical industry, digitalization involves developing and implementing digital technologies at all levels of pharmaceutical operations. The aim is to transform the industry by capturing, analyzing, and using vast amounts of data collected from a wide range of sources to support research and development (R&D), clinical development, drug manufacturing, supply chain management,...