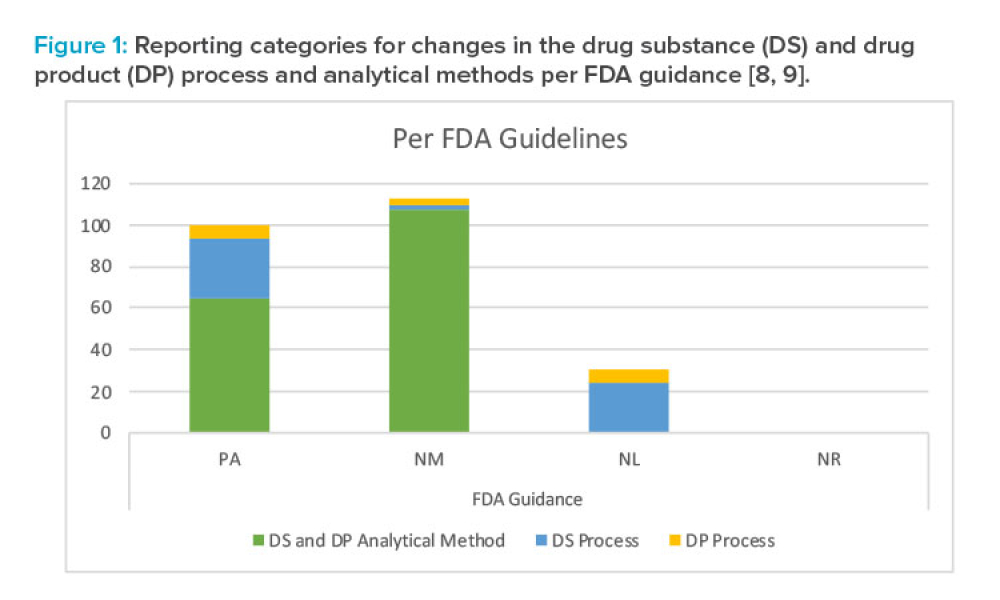
As the demand for accelerated access to medicines expands globally, the pharmaceutical industry is increasingly submitting regulatory applications in multiple countries simultaneously. As a result, Boards of Health (BoHs) are challenged with approving these applications in an accelerated timeframe and accommodating the submission of postapproval chemistry, manufacturing, and controls (CMC)...
Roger Nosal

Related Articles
When working with the common technical dossier (CTD), the structure of Module 2 “follows the scope and outline of the Body of Data in Module 3,”
ISPE is launching a new program, Enabling Global Pharma Innovation: Delivering for Patients in support of many regulatory agencies’ ambitions to promote introduction of innovative pharmaceutical manufacturing. It is incumbent for industry to modernize and innovate pharmaceutical manufacturing to improve efficiency and increase confidence in quality assurance for the benefit of...
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline on Registration of Pharmaceuticals for Human Use (M4)
Virtually every ISPE member has at least one story to tell about how health authority inspections or the review and approval of regulatory applications have affected their efforts to supply critically needed medications to patients globally. Although these stories may emphasize the considerable challenges that ISPE members face, they also frequently identify great opportunities for innovation...