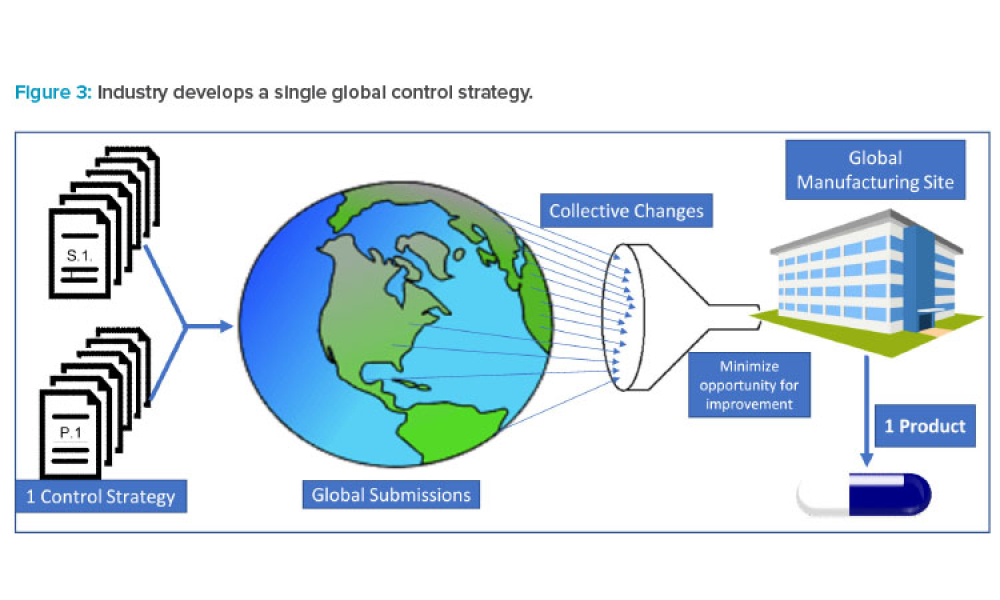
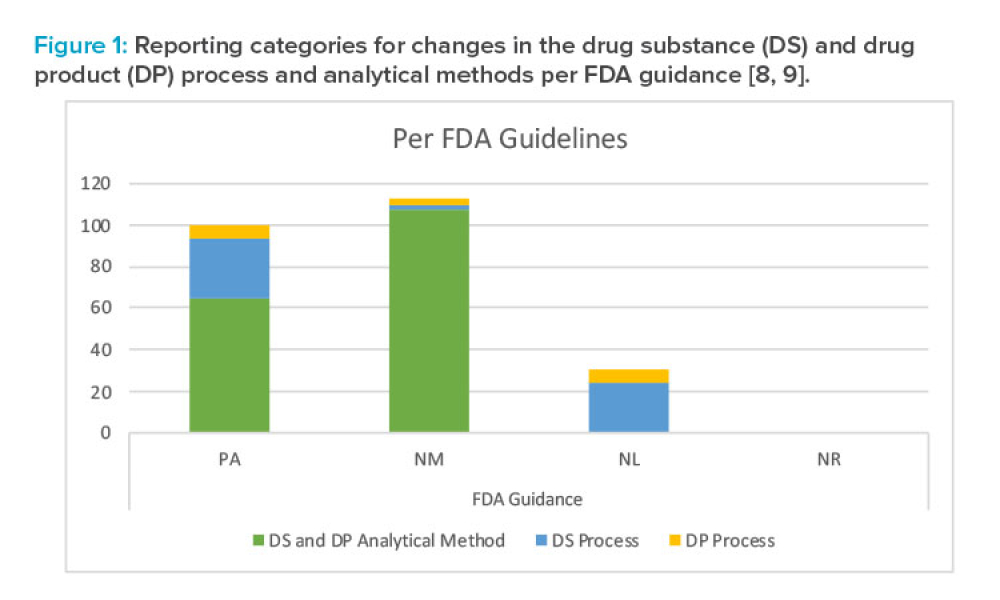
As the demand for accelerated access to medicines expands globally, the pharmaceutical industry is increasingly submitting regulatory applications in multiple countries simultaneously. As a result, Boards of Health (BoHs) are challenged with approving these applications in an accelerated timeframe and accommodating the submission of postapproval chemistry, manufacturing, and controls (CMC)...
Timothy Watson

Gilead Sciences
Vice President – Head of CMC Regulatory Affairs
Dr. Timothy Watson, Ph.D. is Vice President-Head of CMC Regulatory Affairs, Gilead Sciences where he is accountable for ensuring “right first time” global approvals of CMC regulatory submissions, and “right to operate” by maintaining the global licensures. In addition, Tim is accountable for shaping global CMC internal and external regulatory environment at Gilead. Prior to Gilead, Tim lead Pfizer' CMC Advisory Office; a group of global Pfizer technical and regulatory experts that provide guidance & direction to project teams to mitigate regulatory risk & integrate CMC RA policy with product strategies; while developing and advocating policy positions (internally and externally) in conjunction with QO. Tim continues to advocate for global regulatory harmonization & mutual reliance serving as a PhRMA representative to many ICH (International Council of Harmonization) Expert & Implementation Working Groups (EWG & IWG) since 2009. Tim continues to serve on the Boards of Directors for the International Consortium for Innovation and Quality (IQ), and BOD for the International Society for Pharmaceutical Engineering. Tim began his career at Marion Merrell Dow/ Aventis (1994) in chemical research and development (CRD) as an API small molecule process chemist.