We've had the opportunity to interview Hilal Yamaner, an active ISPE Emerging Leader and member of ISPE Women in Pharma®. Throughout this member spotlight, she shares her journey with ISPE, her perspective and passion for AI integration, the work she’s put in to planning a related ISPE Women in Pharma panel session at the upcoming
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
A recently released guidance document, the ISPE Guide: 503A Compounding – Regulatory Basis and Industry Good Practices for Pharmacies, summarizes and contextualizes key information from US governmental bodies relevant to...
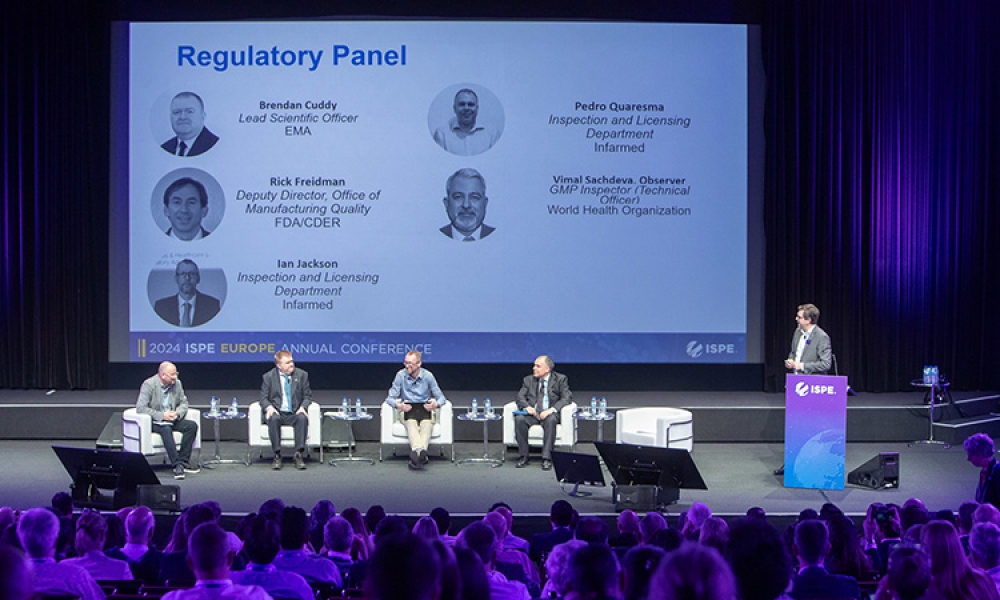
On 17 April 2024 at the 2024 ISPE Europe Annual Conference in Lisbon, Portugal, a panel discussion titled “Harmonization to Break Down Barriers to Robust Supply Chains” featured an international panel of experts who discussed drug recalls, drug shortages, risks that can lead to poor drug product quality, quality risk management, Annex 1 implementation and its effect on the supply chain,...
Eli Lilly has recently achieved a remarkable milestone with the completion of its new synthetic peptide manufacturing facility/platform at its facility in Kinsale, Ireland. This cutting-edge project not only enhances production capabilities but also exemplifies a commitment to innovation and safety. Below is an overview which delves into the details of this groundbreaking achievement and...
ISPE and the ISPE Foundation mourn the loss of a beloved member, mentor, and leader.
Building off the success of the 2023 ISPE Annual Meeting & Expo, the program committee has developed a robust workshop program, taking place Sunday, 13 October, which is based on the practical application of the ISPE Baseline Guide and ISPE Good Practice Guide (GPG) Series, covering the following key areas:
Novel therapies refer to innovative and often groundbreaking approaches to treating medical conditions. These therapies typically involve new modalities aiming to improve upon existing treatments or to provide entirely new options for patients. The development of novel therapies is not immune to the challenges of standard therapeutic pursuits; however, the use of automation can significantly...
In the bustling city of Boston, amidst the vibrant biotech community, the 2024 ISPE Biotechnology Conference will gather industry leaders and innovators for a pivotal set of discussions on Track 4: Lifecycle...
The ISPE Advancing Pharmaceutical Quality (APQ) program team has been actively engaged with the US Food and Drug Administration’s (US FDA) Quality Management Maturity (QMM) program since its inception and along the...
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) has recently announced the adoption of the revised guideline
The outlook for the biopharmaceutical market is promising, with expectations that the market will double in the next 10 years, resulting in new therapies and advances in biopharmaceutical manufacturing. This doesn't sound like much at first, but if you consider how the market has developed over the last 20 years, a doubling in the next 10 years is very significant.
The following blog post was provided by Peyton Myers, an undergraduate student at Appalachian State University. Myers attended the 2023 ISPE Annual Meeting & Expo in Las Vegas as an ISPE Foundation Professional Development Grant recipient.
The integration of data science in biopharmaceutical manufacturing, emphasizing data quality, tech transfer efficiency, and process optimization, is the heart of this track. Led by industry experts, discussions explore leveraging digital twins, predictive analytics, and continuous improvement initiatives. Additionally, interactive roundtable discussions provide attendees with a dynamic forum...
The pharmaceutical sector stands at a crossroads of immense possibilities. We are witnessing an unprecedented surge in innovation, fueled by a remarkable partnership between industry and regulatory authorities. For example, there are initiatives fostered by the US Food and Drug Administration (US FDA) to promote innovation with programs such as CATT (CBER Advanced Technologies Team) and ETP...
Through the ISPE Foundation Professional Development Grant program, Silas Tamufor attended the 2023 ISPE Annual Meeting & Expo in October 2023. Tamufor is a PhD student and ISPE Boston Chapter member who began serving as the ISPE Boston Educational Programs Committee Chair in December 2023. He, along with 87 other students and recent graduates, attended the conference thanks to the...
To meet the biopharmaceutical industry’s duty to manufacture safe and effective therapies for patients, a robust quality system is fundamental to success. A quality system should link to quality culture and prioritize focusing on quality, led by management, that fosters sustainable compliance and consistent production of high-quality drugs. Strong quality culture attributes include a proactive...
Stability sampling and testing are key to ensuring that products maintain safety, identity, strength, purity, and quality throughout their claimed shelf life. It is also a regulatory requirement per ICH Q5. However, storing product samples in different environmental conditions, testing those samples for three to five years (or more) after initial manufacture, and properly analyzing and...
ISPE hosted more than 450 attendees in person and virtually for the 2024 ISPE Aseptic Conference in Vienna, Austria. Keynotes and education sessions provided a comprehensive overview of key topics and trends...