
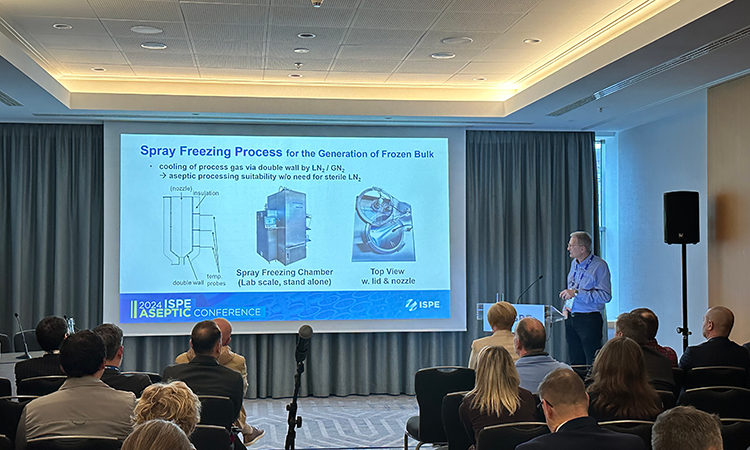
Thank you to all who made the 2024 ISPE Aseptic Conference such a big success!










In the intricate world of pharmaceuticals, where precision and safety are paramount, the marriage of innovation and sustainability is not just an aspiration—it's a necessity. Picture a scenario where the pursuit of product purity meets the responsibility to our planet. Welcome to the realm of "Eco-friendly Pharma: Sterilization and Sustainable Practices." As we embark on this exploration,...
As the 2024 ISPE Aseptic Conference Chair, Christa Myers, Sr. Fellow - Aseptic and Sterile Products, Vertical Market Leader at CRB gets asked many times: what’s next? What are the new trends? Can Aseptic manufacturing change be easier, more cost-effective, and manage risks better? What are the inspectors looking for? Why can this not be more clear? Let’s find those answers together.
In the pharmaceutical industry, which is highly regulated, aseptic processing is a critical component that ensures the sterility of products. Regulators have a set of comprehensive requirements that minimize the risk of contamination. Regulators set the requirements; however, the industry has an obligation to the patients who rely on and expect a drug that is safe and free of contamination....
Annex 1 has been in effect since August 2023. But what has been its impact? This topic will be discussed at the 2024 ISPE Aseptic Conference in Vienna, Austria and virtually. Speakers from both industry and...
The 2024 ISPE Aseptic Conference will offer a glimpse into the best discussions on the future of aseptic manufacturing. Attendees will learn how optimization is evolving both in the form of digitalization and in...
In the intricate world of pharmaceuticals, where precision and safety are paramount, the marriage of innovation and sustainability is not just an aspiration—it's a necessity. Picture a scenario where the pursuit of product purity meets the responsibility to our planet. Welcome to the realm of "Eco-friendly Pharma: Sterilization and Sustainable Practices." As we embark on this exploration,...
As the 2024 ISPE Aseptic Conference Chair, Christa Myers, Sr. Fellow - Aseptic and Sterile Products, Vertical Market Leader at CRB gets asked many times: what’s next? What are the new trends? Can Aseptic manufacturing change be easier, more cost-effective, and manage risks better? What are the inspectors looking for? Why can this not be more clear? Let’s find those answers together.
In the pharmaceutical industry, which is highly regulated, aseptic processing is a critical component that ensures the sterility of products. Regulators have a set of comprehensive requirements that minimize the risk of contamination. Regulators set the requirements; however, the industry has an obligation to the patients who rely on and expect a drug that is safe and free of contamination....
Annex 1 has been in effect since August 2023. But what has been its impact? This topic will be discussed at the 2024 ISPE Aseptic Conference in Vienna, Austria and virtually. Speakers from both industry and...
The 2024 ISPE Aseptic Conference will offer a glimpse into the best discussions on the future of aseptic manufacturing. Attendees will learn how optimization is evolving both in the form of digitalization and in...
In the intricate world of pharmaceuticals, where precision and safety are paramount, the marriage of innovation and sustainability is not just an aspiration—it's a necessity. Picture a scenario where the pursuit of product purity meets the responsibility to our planet. Welcome to the realm of "Eco-friendly Pharma: Sterilization and Sustainable Practices." As we embark on this exploration,...
As the 2024 ISPE Aseptic Conference Chair, Christa Myers, Sr. Fellow - Aseptic and Sterile Products, Vertical Market Leader at CRB gets asked many times: what’s next? What are the new trends? Can Aseptic manufacturing change be easier, more cost-effective, and manage risks better? What are the inspectors looking for? Why can this not be more clear? Let’s find those answers together.
