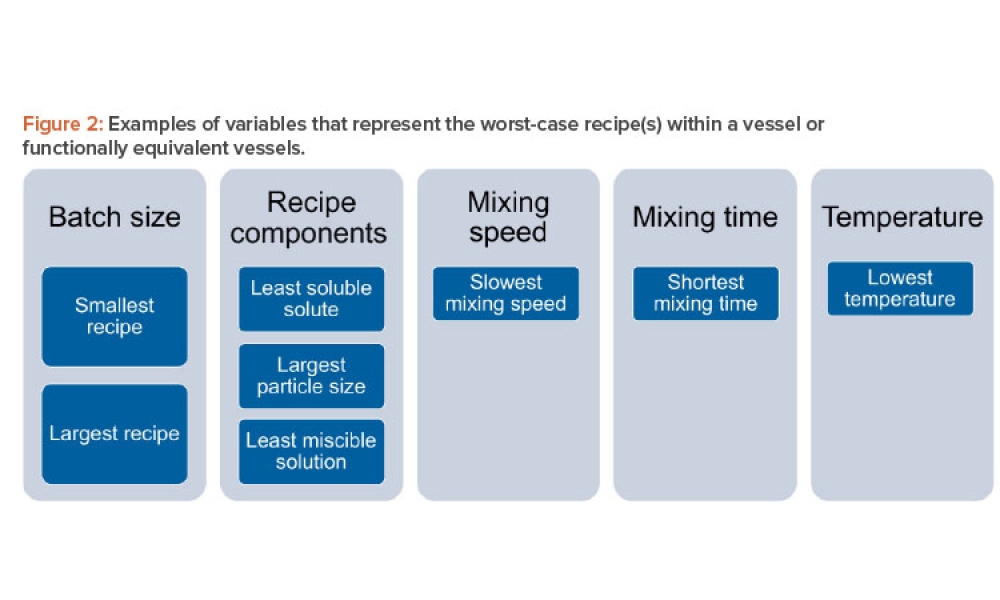
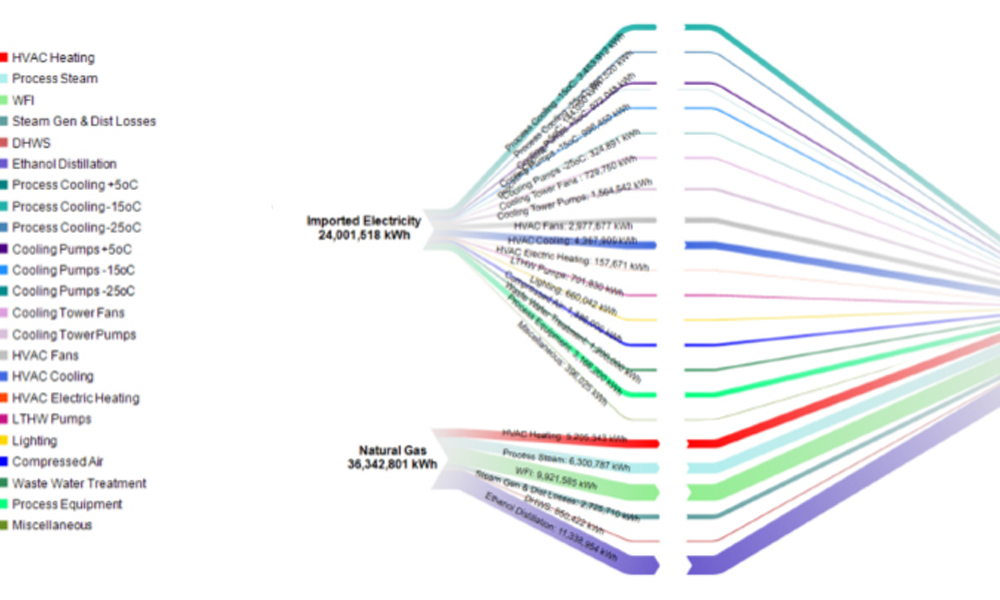
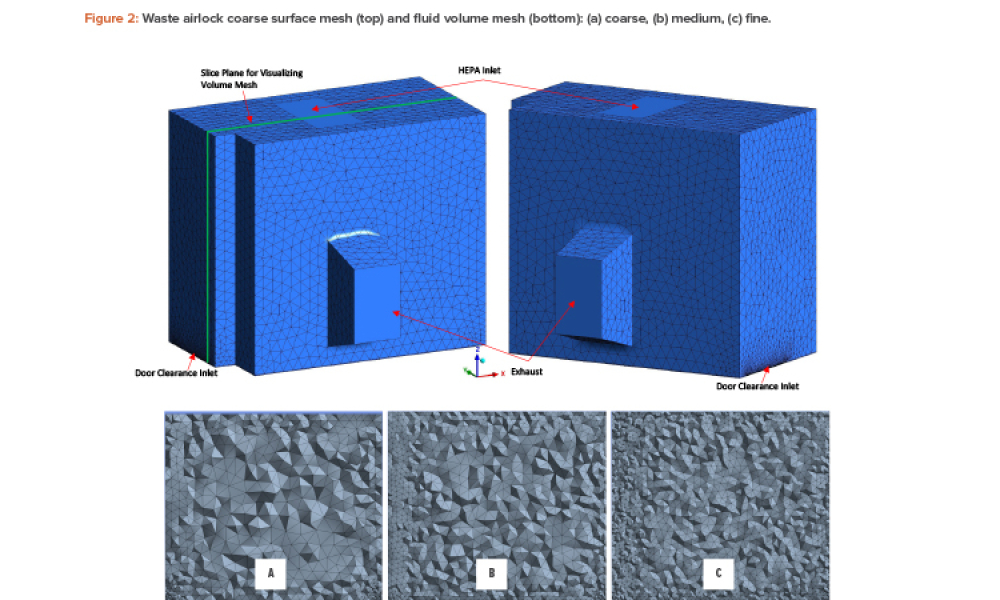
Microbiological and viral contamination control (Microbio Contam Ctrl) refers to the non-intended or accidental introduction of infectious material like bacteria, yeast, mold, fungi, virus, prions, protozoa, or their toxins and by-products.
Join the Conversation
As an ISPE member you can engage with 22 active CoPs, including new communities focused on Artificial Intelligence and Sustainability. Connect with experts and join the conversations: Become an ISPE member
ISPE members: Get more involved by volunteering.
Guidance Documents
Containment (3)
+Critical Utilities (5)
+Manufacturing Operations (3)
+Microbiological & Viral Contamination Control (16)
+Oral Solid Dosage (1)
+Quality Control (2)
+Quality by Design (1)
+Regulatory (3)
+Sterile Products (1)
+Sustainability (1)
+Sustainable Facilities, HVAC, & Controlled Environments (3)
+Validation (1)
+Community Discussions
Community Discussions
Aug 18, 2025
Aug 18, 2025
Quality Assurance
Aug 11, 2025
Quality
Lifecycle Management
Validation
Jul 25, 2025
Data Integrity
Jul 24, 2025
Information Systems
Artificial Intelligence
Data Integrity
Jul 17, 2025
Manufacturing Operations
Oral Solid Dosage
Jul 08, 2025
Pharma 4.0™
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
Hands-On Aseptic Processing & Annex 1
+Aseptic Processing & Annex 1 Training Course
+HVAC & Environmental Control for Life Science Facilities Training Course
+Cleaning Validation Principles Training Course
+CIP System Design, Integration and CIP Chemicals Training Course
+Featured Conferences

White Papers
July / August 2025
The AI Paradox Cover: The increasing digitalization of the pharmaceutical and medical device…
May / June 2025
The ATMP Issue: In this issue, we focus on the manufacturing of advanced therapy medicinal products…
March / April 2025
A Skill Management Framework for a Pharma 4.0™ Workforce Feature: Pharma 4.0™ is driving fundamental…