The New ISPE Discussion Paper, “Unique Identification on Primary Containers to Drive Product Traceability & Quality” explores how container traceability...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Over more than two decades, the pharmaceutical industry has increasingly adopted single-use technologies primarily to simplify manufacturing while hoping to reduce cost; however, the need to reduce environmental burden continues to have a huge impact on these operations. As a member of the 2021 ISPE Aseptic Conference Program Committee I am privileged to be leading the session titled:...
Teresa Minero has served as ISPE Italy Affiliate Chair, Vice-Chair, and as a leader of the Affiliate’s Student and Women-in-Pharma Groups. An ISPE Member for more than 26 years, she is currently a member of the ISPE Board of Directors. The Founder and CEO of her own company, LifeBee, Teresa has numerous accomplishments,...
What is accountability? By definition, “it is taking or being assigned responsibilities for something that you have done or something that you are supposed to do.” We can take this far beyond that. Accountability means doing what we say we will do, always, on time and to the best of our ability. It means setting goals and achieving them. It means consideration of others, saying yes to things...
The 2021 ISPE Aseptic Conference is your key to unlocking success – success in personal technical growth, success in a robust project execution, and success for continuous operation and improvement. I have said for...
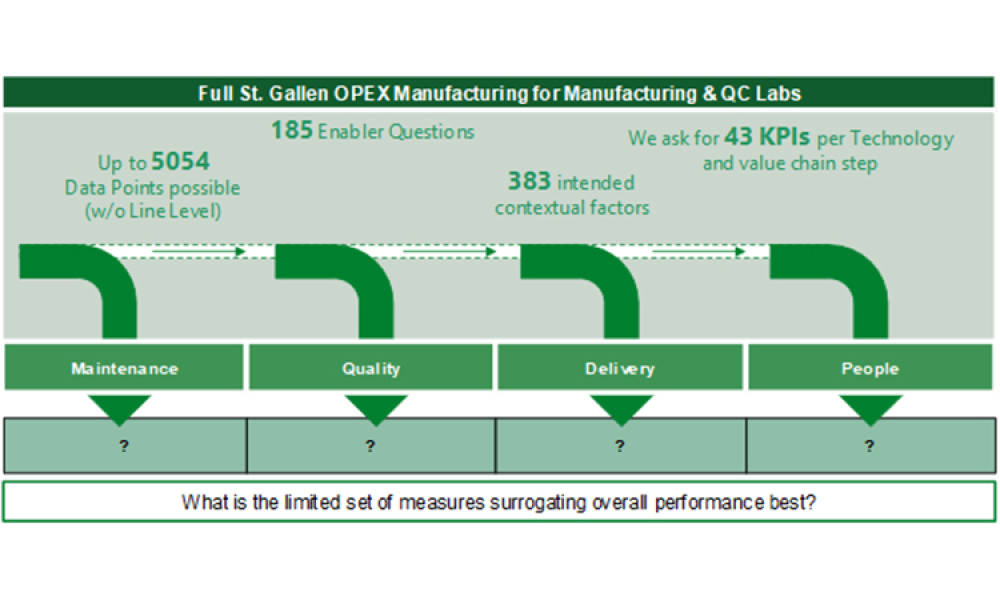
The ISPE APQ Assess, Aspire, Act and Advance Framework will be complemented with an OPEX Benchmarking and ICH Q10 maturity assessment tool specifically developed for use with the APQ program by the University of St.Gallen, Switzerland. Used in conjunction with the APQ self-assessment tools for quality management maturity, the pre- and post- benchmarking activities offer objective evidence of...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from December 2020. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
As we enter the new year, this is the perfect time to make the 2021 ISPE Aseptic Conference part of your personal professional development plans. The virtual conference will allow participation from across the globe...
Lou Kennedy, Nephron Pharmaceuticals’ President & CEO and active ISPE Member, was recently honored with a 2020 Business Leader and Public Servant of the Year Award. We spoke with Lou to learn more about her company, the many initiatives she and Nephron are undertaking on behalf of pharma, Nephron’s community, and how women are making a difference in the industry.
The 2021 ISPE Aseptic Conference 30th Anniversary will be held on 15 – 17 March offering attendees several unique perspectives and case studies across two separate tracks. For many years, the ISPE Aseptic Conference...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from November 2020. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
It is time to celebrate the 30th anniversary of the ISPE Aseptic Conference at the 2021 ISPE Aseptic Conference. Hard to believe, isn’t it?
The Life Sciences industries adhere to strict documentation practices to prove quality and compliance. We’re all familiar with the truism “If it isn’t written down, it didn’t happen.”
FDA Commissioner Stephen M. Hahn, MD, spoke about the lessons learned from COVID-19 in a presentation during the ISPE Annual Member Meeting on 5 November at the 2020 ISPE Annual Meeting & Expo. He discussed how the FDA has been responding to the pandemic, including how actions taken in response to the pandemic have been combined with FDA knowledge from prior public health crises to...
2020 ISPE Europe Annual Conference
This article covers a regulatory panel discussion at the virtual 2020
We are constantly being influenced and we ourselves are influencers. As children, we hear the quote that “sticks and stones may break my bones, but words will never hurt me.” What people say and even what people do not say, can have a lasting impact. We are first influenced by our families and some people’s careers follow their parents, grandparents, siblings, and other relatives’ footsteps. I...
2020 ISPE Europe Annual Conference
At the 2020 ISPE Europe Annual Conference on 16–17 September, which was a virtual conference, three regulatory panel discussions included more than 20 regulators...
Article 117 of the EU Medical Device Regulation (MDR), fully applying May 26, 2021, is significantly impacting BioPharma companies. While integral drug-device combination products (such as pre-filled syringes and pre-filled injectors) are regulated as medicinal products in Europe (EU), certain aspects of the Medical Device Regulation apply to the device component of the product.