Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
At ISPE, our commitment to advancing the pharmaceutical industry has always revolved around delivering gold-standard Guidance Documents — a trusted source of information that’s been a helping hand for professionals like you to rely on when forging new paths and exploring uncharted territory . In the ever-evolving landscape of pharmaceutical and scientific advancements, we understand that how...
The 2023 Facility of the Year Awards (FOYA) Social Impact category recognizes companies that accelerated a shift to sustainable facility design, intended to ensure the effective use of energy, minimize waste, reduce carbon footprint, incorporate green manufacturing techniques, and reduce environmental...
Few industries have as deep a footprint in California, USA as life sciences. The sector is home to nearly 14,000 businesses that employ more than a million people at an average wage of more than $162,000 – Californians employed in life sciences earn a staggering $54.6 billion a year. And despite uncertainties in the US economy and massive layoffs in the high-tech sector, California life...
For several years, ISPE has steadfastly organized hands-on training sessions to enrich classroom learning. Despite the challenges posed by the pandemic, we have successfully developed a program that integrates practical, experiential learning, leveraging ISPE's comprehensive training materials. We are thrilled to announce our partnership with the European Aseptic and Sterile Environment (EASE)...
PIC/s Annex 1, and the WHO Annex 2 for the manufacturing of sterile products took effect on 25 August 2023, and the time to attend the 2023 ISPE Pharma 4.0™ and Annex 1 Conference couldn’t be better. The Equipment Innovation and Annex 1 Implementation Track Co-leads Richard Denk and Matthew Gorton and the Emerging Leader Co-lead Pol Bonet invite you to join them for this exciting event in...
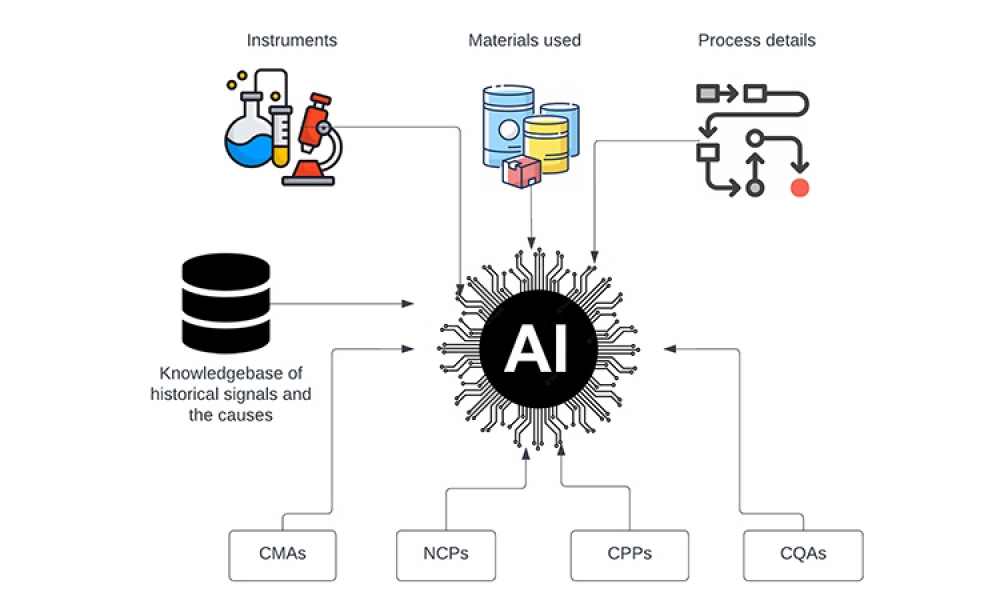
The pharmaceutical industry is constantly evolving, placing a significant emphasis on emerging technologies that facilitate quicker access of medications for patients globally. This unceasing drive for innovation and improvement has led the industry to embrace cutting-edge technologies like data analytics, machine learning, and artificial intelligence. These technologies are not just trends;...
With a recent increase in interest and participation, the ISPE GAMP® Special Interest Group (SIG) focusing on Manufacturing Execution Systems (MES) has mapped out an ambitious number of subject areas to use as the basis for future publications, presentations, and best practice guidance documents. With the co-leadership of Christian Wöelbeling, Executive Industry Advisor at Körber Pharma...
Facility of the Year Award (FOYA) Winners in the Supply Chain category exemplify novel application of principles, systems, and management tools aimed at improving operational speed, robustness, and response. These could be applied to areas in which delivery to market impacts patients, technology amplifies supply...
Danielle Gabrish is the Director of Clinical Biologics at AstraZeneca and the Chair of the ISPE Women in Pharma® group for ISPE’s Chesapeake Bay Chapter. She’s also a mom, mentor, and advocate for women and Emerging Leaders.
You may have already read through our Members Guide to ISPE’s 2023 Annual Meeting & Expo, which offers a high-level overview of all the conference highlights. Within this article, we’ll break down the various Women in Pharma® activities scheduled throughout the meeting. From an empowering self-defense class to a nostalgic...
Addressing the unmet healthcare & medical needs is among the top priorities for global governments and organisations, including the pharmaceutical industry. We are facing unprecedented challenges due to the change with medical knowledge doubling every 72 days
Shanghai ISPE Pharmaceutical Information Company (SPIC) held the 2023 Global Biopharma Engineering Forum and ISPE China Conference in Hangzhou, China on 26-27, May 2023. Mr. Ping Zhang, Chairman of SPIC and the ISPE China Committee, introduced ISPE and provided an in-depth analysis of the prospects of domestic and foreign bio-pharmaceutical enterprises. The in-depth discussions from this...
Facility of the Year Award (FOYA) Winners in the Innovation category exemplify the application of novel manufacturing processes, innovative design concepts, new technologies, and unique solutions that...
Cloud-based systems for plant process management (PPM)
Facility of the Year Award (FOYA) Winners in the Operations category exemplify the application of novel tools and approaches to delivering projects that improved efficiencies, overcame unusual challenges,...
Building off of the success of the 2022 ISPE Annual Meeting & Expo, the 2023 ISPE Annual Meeting & Expo Program Committee has developed a robust Workshop program covering key areas in Digital Transformation (DT) and Pharma 4.0™, Quality Risk Management (QRM), Contamination Control Strategy (CCS) and Annex 1, Facility...
Data Governance is currently utilized in many areas of business and government including finance, banking, telecommunications, and by the agencies regulating these industries. In pharmaceuticals, digital validation is still at a relatively early stage of the digital transformation journey where data governance is currently managed in silos of data (Industry 3.0) and the industry has of yet to...