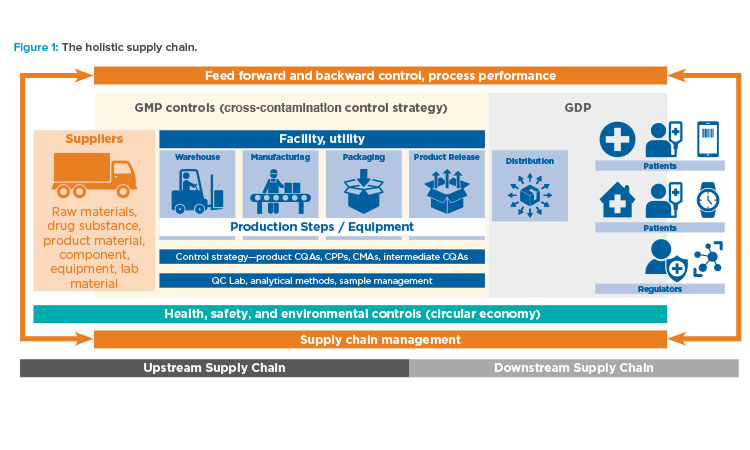
Read, Learn, Innovate: Pharmaceutical Engineering® Articles on Pharma 4.0™
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are some of the many articles published about Pharma 4.0™. Now is a great time to catch up with Pharma 4.0™, especially with the upcoming 2022 ISPE Pharma 4.0™ and Annex 1 Conference in Vienna on 7–8 December.