Content focuses on the financial impact of data management systems on drug development, manufacturing, and distribution; the basic computer system life cycle model and the activities and software quality assurance practices in each phase; and the controls and methods necessary to maintain data integrity and security.
Guidance Documents
Advanced Manufacturing (1)
+Artificial Intelligence (1)
+Commissioning & Qualification (1)
+Data Integrity (17)
+GAMP® (15)
+Knowledge Management (1)
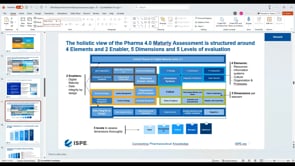
+Pharma 4.0™ (1)
+Process Analytical Technology (1)
+Quality Assurance (1)
+Quality by Design (1)
+Regulatory (1)
+Validation (13)
+Community Discussions
Community Discussions
Jul 17, 2025
Manufacturing Operations
Oral Solid Dosage
Jul 08, 2025
Information Systems
Artificial Intelligence
Data Integrity
Jul 08, 2025
Pharma 4.0™
Jun 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Jun 19, 2025
Quality
Lifecycle Management
Validation
May 22, 2025
Pharmaceutical Engineering Magazine Articles
White Papers
January / February 2025
GAMP® is indispensable for safeguarding the safety, quality, and compliance of pharmaceutical…
January / February 2024
Stakeholders across industries are becoming accustomed to using information technology (IT) systems…
March / April 2022
This article aims to refresh information on open-source software (OSS) within regulated computerized…

Webinars
Upcoming
On-Demand
ISPE in the News
Latest
-
Double Your Impact: ISPE Join/Renew Offer
-
New Certificate Programs from ISPE Academy
-
Automated Drug Discovery Facility Opens in Scotland
-
US FDA Seeks Comments on Draft Guidance for Cancer Drug Combinations
-
Lupin API Plant Earns GMP Certification from Australia's TGA
-
Moderna Shelves Plan for mRNA Plant in Japan
-
GenesisM Starts Work on Biomanufacturing Facility in Massachusetts
-
Overcoming Barriers to Advanced Manufacturing
-
Experts Discuss Pharma Supply Chain Resilience
- ISPE Webinar: Life Cycle Approach to Process Validation - Stage 3 Implementation