Released in November 2021, the ISPE Guide: Advanced Therapy Medicinal Products (ATMPs)–Autologous Cell Therapy focuses on manufacturing facility development and design for autologous cell therapies for...

Downloads
Viral Vector Platforms: Intersection of Facility And Program
Cover: Realizing the promise of any novel viral vector therapeutic depends on the innovator’s ability to constantly meet evolving program requirements set in the product’s preclinical; clinical; chemistry, manufacturing, and controls; and market strategies. A key enabler to success is establishing a robust yet nimble viral vector manufacturing platform that delivers high-quality product on time and in full while managing costs.
ATMP Facilities: Adapting to a Multimodal Future
Feature:In a recent advanced therapy medicinal products innovator survey, two-thirds of respondents reported that they are developing multiple drug platforms. Without a guarantee of commercial success for any single product, many companies are making smart choices by creating new therapies to tackle di erent diseases and mitigate risk.
FOYA Winners Share Lessons Learned in Facilities Development
Feature:What do recipients of ISPE’s prestigious Facilities of the Year Award (FOYA) know that has helped their projects succeed? What are the lessons learned from achievements in facilities development, including forward-looking projects that encompass and inspire changes in the industry? Pharmaceutical Engineering® spoke with nine FOYA winners from recent years about the lessons they learned and the advice they have for those challenged with building a new facility or renovating an existing one.
Special Report: Operation Warp Speed: A View From the Inside
Special Report:Operation Warp Speed coordinated US government support of the pharmaceutical industry’s e ort to develop and deliver vaccines and therapeutics across the US to fi ght the COVID-19 pandemic. This article provides an inside look at the work done by this team to address the threat posed by COVID-19.
Special Report: Pandemic Progress: Industry’s Journey From 2020 to Today
Special Report:In 2020, the world was grappling with the SARS-CoV-2 virus. In two years, there are multiple vaccines and treatments along with great knowledge about the virus—and about how the industry mobilized, partnered, and achieved tremendous strides in addressing the global pandemic.
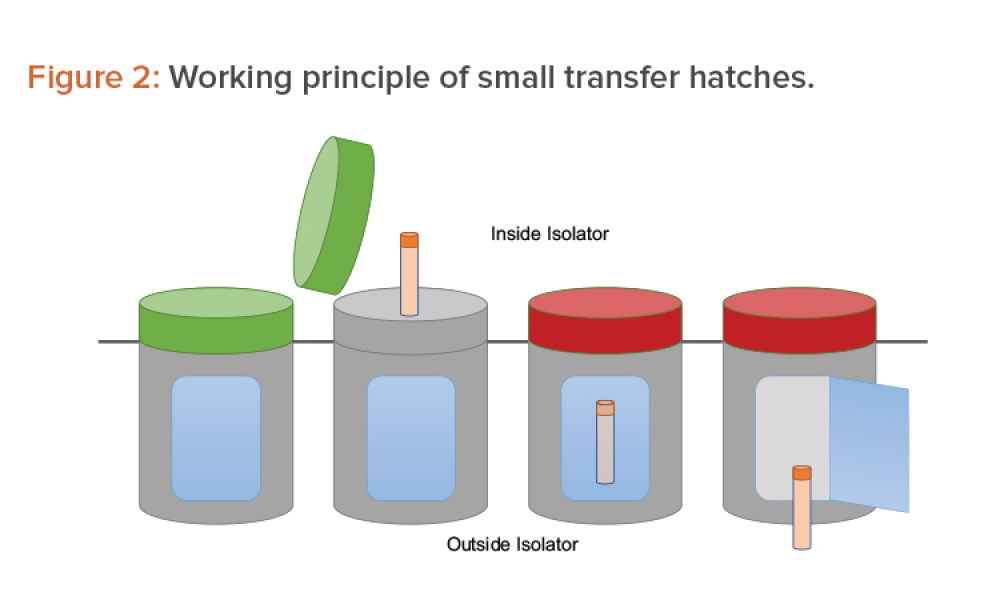
Moving From Cleanroom to Isolation Technology for ATMPs
Technical:manufacturing challenges beyond those typically addressed by pharmaceutical chemistry. This article analyzes the possibility of transferring a cell and gene therapy process from the cleanroom approach to an environment based on isolation technology or, in other words, of moving the process from an open space manipulation to a closed and segregated space concept.
In This Issue
In a recent advanced therapy medicinal products (ATMPs) innovator survey, two-thirds of respondents reported that they are developing multiple drug platforms.
What do recipients of ISPE’s prestigious Facilities of the Year Award (FOYA) know that has helped their projects succeed? What are the lessons learned from achievements in facilities development, including forward-looking projects that encompass and inspire changes in the industry? Pharmaceutical Engineering® spoke with nine FOYA winners from recent years about the lessons they learned and...
Two sessions at the 2022 ISPE Facilities of the Future Conference in early February captured varied views of emerging technologies in the pharmaceutical industry, and the industry’s work to embrace...
The life cycle approach to process validation stresses the need for continued monitoring of process performance to ensure that the manufacturing process remains stable and predictable, i.e., in a state of control. This life cycle stage is known as continued process verification (CPV) or ongoing process verification (OPV).
The unprecedented speed in the development and global rollout of vaccines and treatments for COVID-19 over the last two years has made it possible to get back to a new normal. The 2022 ISPE Facilities...
As a part of taking care of yourself, one must risk something. For me, taking a risk can be scary. I get nervous, anxious, and, at times, begin to doubt myself. Then I remember why I’m taking a risk and what the potential rewards will be, and confidently move forward with my decision.
What do innovating new therapies, surviving the start-up phase of a company, and entering an industry workforce have in common? All three of these, if successful, demonstrate resilience.
Operation Warp Speed coordinated US government support of the pharmaceutical industry’s effort to develop and deliver vaccines and therapeutics across the United States to fight the COVID-19 pandemic. This article provides an inside look at the work done by this team to address the threat posed by COVID-19.
In 2020, the world was grappling with how to slow the spread of the SARS-CoV-2 virus and appropriately treat people who had the COVID-19 infection without approved therapies or vaccines. In two years, there are multiple vaccines and treatments along with great knowledge about the virus—and about how the industry mobilized, partnered, and achieved tremendous strides in addressing the global...
The lack of diversity in the pharmaceutical engineering industry is widely recognized. Less well understood is why change is so hard to achieve. From my years of work in this space, and through observation of ongoing efforts to embrace diversity in all forms, I have developed a hypothesis: Progress is stifled because we as a society wrongly believe diversity and inclusion efforts are...
Advanced therapy medicinal products (ATMPs) pose specific manufacturing challenges beyond those typically addressed by pharmaceutical chemistry. Often in current ATMP applications, a change in approach is introduced at some point in the development process out of convenience or necessity, which then results in a change in technology. This article analyzes the possibility of transferring a cell...
Heightened awareness, due to the pandemic, of the need for domestic manufacturing capacity has rejuvenated the biopharmaceutical manufacturing industry and resulted in new commissioning projects. However, cross-country/continental travel restrictions and social distancing–based work protocols during the first two years of the pandemic necessitated adopting unique commissioning approaches....
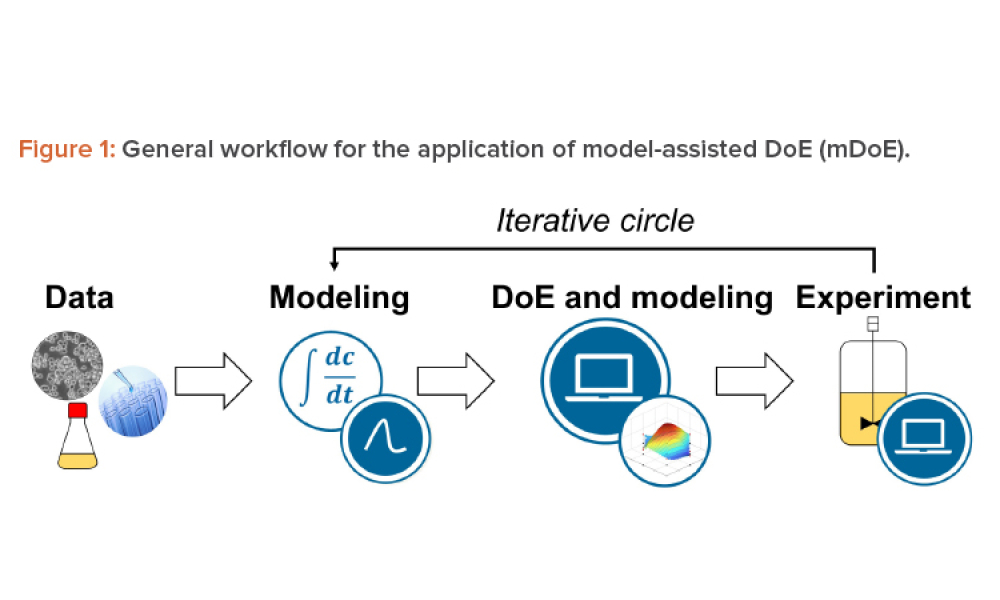
The implementation of a mammalian cell-based biopharmaceutical manufacturing process demands robust methods for knowledge handling, from early-stage development and technology transfer to production scale. Mathematical process modeling can summarize this knowledge as the relationships of critical quality attributes to critical process parameters using mathematical equations and sound...
Realizing the promise of any novel viral vector therapeutic depends on the innovator’s ability to constantly meet evolving program requirements set in the product’s preclinical; clinical; chemistry, manufacturing, and controls (CMC); and market strategies. A key enabler to success is establishing a robust yet nimble viral vector manufacturing platform that delivers high-quality product on time...