Industry Perspective: Validation 4.0 - Shifting Paradigms

As Pharma 4.0™ increasingly becomes reality, our validation practices must change. We can no longer apply 20th-century thinking to 21st-century technology and resources. Validation must adapt to industry shifts from iterative to disruptive innovation, from batch to continuous processing, from bulk processing to personalized medicine, from centralized systems to the Internet of Things (IoT), from controlled data to distributed data, and similar changes.
What does validation in the context of Pharma 4.0™—i.e., “Validation 4.0”—look like, and why do we as validation professionals care? Just as validation practices and paradigms shifted throughout the industry’s prior evolution, so must they change to keep pace with future evolution. Adoption of quality risk management (QRM) and quality by design (QbD) principles and practices in validation lagged behind industry adoption. Unless we prepare now, the adoption of validation practices for Pharma 4.0™ innovations will lag behind industry adoption, and this could jeopardize implementation of industry innovations. This challenge applies to all validation, not only computer system validation.
Lessons from Other Industries
The 20th century was the era of blockbuster pharmaceuticals, during which the pharmaceutical industry unsurprisingly adopted the principles of mass production. As we enter the era of product differentiation and personalized medicine, we should learn from other industries that started this journey before us.
After the 2001 recession, the semiconductor industry went through a fundamental transition through which it was transformed in a matter of 10 years from high-profit, high-waste operations to one of the world’s most highly automated, lean industries. This astounding rate of adoption was facilitated by what we know as Industry 3.0 computer technologies.
In the 1990s, the aerospace industry embarked on an initiative to digitize product information to alleviate costly and burdensome regulatory and customer documentation requirements. Each F-16 jet fighter delivered was rumored to require a volume of documentation sufficient to fill a 747 jumbo jet. This initiative resulted in the product life-cycle management (PLM) systems that are now commonplace in the aerospace industry.
The automotive industry delivers, with a few hiccups, a very high level of quality with little regulatory oversight, because quality is understood to be a competitive advantage, and therefore a critical business goal, in this industry. The relevant concept that the automotive industry has adopted is QbD, an integrated product design approach, which is also the cornerstone of the Pharma 4.0™ holistic control strategy.1
Since the 1990s, the automotive, aerospace, and defense industries have used concurrent engineering principles that are enabled by digital product models, or model-based design. At the heart of this process are structured data models of the product aided by software tools that allow multiparty collaboration on product design, production, and testing.
These industries are already primed to be able to adopt artificial intelligence/machine learning or augmented reality/virtual reality because these tools can build on the data foundation they already have. In contrast, most of the pharmaceutical industry lags behind other industries in applying concepts such as digital maturity, digital twins, PLM, and QbD.
Validation and Pharma 4.0™
What does this mean for validation? Ultimately, the community of validation professionals must ask ourselves two questions:
- Can we adequately deal with the pace and complexity of pharmaceutical evolution and paradigm shifts by applying current methodologies?
- Can we build on our current methodologies, or must those methodologies change to accommodate industry evolution and paradigm shifts?
Enablers and Challenges
There are three key external enablers and trends for a new Pharma 4.0™ approach to validation:
- Regulatory encouragement to help the pharmaceutical manufacturing sector mature to a maximally efficient, agile, and flexible state, in which manufacturers reliably produce high-quality drugs without extensive regulatory oversight (21st-century GMP initiative)
- Advancement of health sciences knowledge to the molecular level, enabling the pharmaceutical industry to evolve from “discovering” medicines to “engineering” the next generation of differentiated, competitive medicines
- Innovative technologies that are smarter and more adaptive in conjunction with software capabilities to handle large amounts of adaptive, self-optimizing data in near real time
In the new digitalized and connected Pharma 4.0™ world, we must be able to adapt to evolution in production, such as from blockbuster to personalized medicine, and respond immediately to changes in consumer demand, supply chain, raw material and product variability, equipment breakdown, and even the way we work (think of the COVID-19 pandemic’s impact). Such responses are only possible if we understand and accept the impact of changes from a holistic perspective, looking at the entire value network that covers the controls that must be in place to ensure the product can be manufactured and supplied the patient.
This is the holistic control strategy, which enables us to predict (or simulate) this impact in real time and propose the necessary process adjustments. The science and risk basis of the predicted outcome of the change will provide a statistical basis to estimate the risks to the patient, product, business, operator, environment, and so on, and to use this risk estimate to determine the next step.
However, current validation strategies are not well suited to this new paradigm. If the simulated change is implemented, current validation paradigms would likely not consider such a process to be in a state of control, particularly if this process were not just predictive, but also adaptive.
How will we manage processes that have automated decisions to change or improve? How will we ensure such a process is validated? Perhaps we will require a completely new meaning of validation for these Pharma 4.0™ self-optimizing or self-decision-making systems and processes.
Process Performance Qualification
Current models for initial and continued process validation assume closed manufacturing processes using algorithm-based input-output automation and control. These models need to change across the entire value network to accommodate the holistic control strategy and manufacturing processes that are distributed—even to the point of single-patient/bedside manufacturing/delivery—and that will apply new technology.
Product and process knowledge derived from process development will be refined using real-time process data. Digital twin technology will be used to provide additional understanding and will contribute to the validation process.
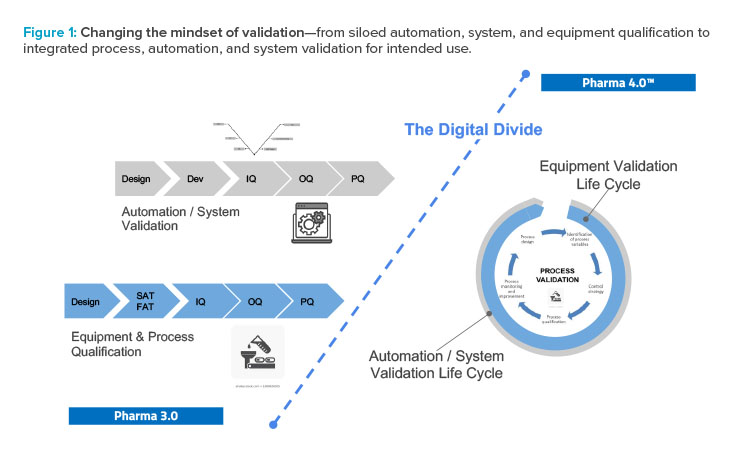
The holistic control strategy will facilitate the delivery of process performance qualification and incorporate a range of innovative technologies. Validation will be built in, and the various groups involved in validation will have to work together. A siloed approached will no longer work (see Figure 1).
How Do We Get There from Here?
Validation concepts have developed and evolved as the industry has tried to adopt new trends and technologies. However, in Pharma 4.0™ we must integrate these concepts; therefore, it is now the time to rethink the validation strategy and facilitate the move to agile processes. The validation strategy must be part of the holistic control strategy, and stakeholders must use critical thinking to ensure lean and robust risk assessment.
It is now the time to rethink the validation strategy and facilitate the move to agile processes.
Key subject matter experts will require experience to set up lean processes. There is an opportunity here for ISPE to help companies improve their digital maturity and move to lean processes as part of the holistic control strategy.
This transition in validation strategies is facilitated by adopting QRM-based integrated commissioning and qualification principles defined by the revised ISPE Baseline Guide, volume 5;2 risk-based process performance qualification, as defined in the Good Practice Guide for Practical Implementation of the Lifecycle Approach to Process Validation;3 and the GAMP 5 Guide: Compliant GxP Computerized Systems.4 Together, these efforts can potentially help shift validation paradigms to make Validation 4.0 a reality.
Conclusion
Current practices lead to silos between computer system validation, facility and equipment qualification, product and process qualification, and the overall quality systems. These silos inhibit innovation within the industry. This is not just a business concern—it is also a risk to the delivery of lifesaving therapies to the patients served by the industry. The goal of Validation 4.0 is to develop a cohesive, harmonized, integrated, holistic, risk-based approach for process performance qualification incorporating computer system validation that builds on the Pharma 4.0™ operating model and includes the holistic control strategy, digital maturity, and data integrity by design. This approach will help support and facilitate current and future innovations in the pharmaceutical industry.










