In this article, potential Pharma 4.0™ technological solutions that can enhance continuous process verification (CPV) 4.0 are discussed. The necessary paradigm shift will allow companies to predict deviations more accurately, perform root cause...
Pharma 4.0 News
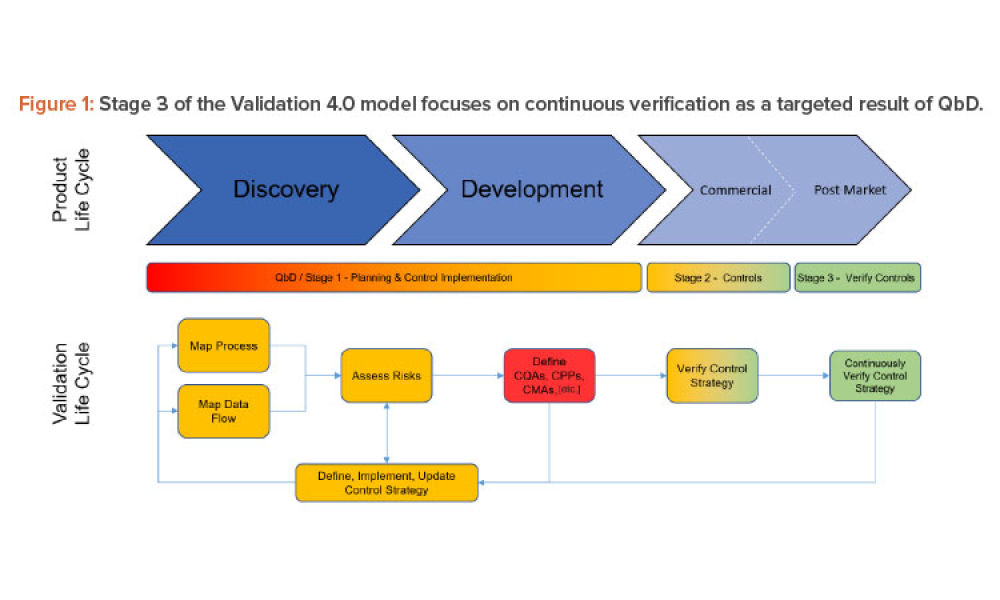
Three case studies on Validation 4.0 demonstrate how quality by design (QbD) principles, when applied with digitization, can verify processes in scale-up and technology transfer, and why blend and content uniformity matter for tablet integrity.
ISPE’s Pharma 4.0™ initiative provides guidance, aligned with the regulatory requirements specific to the pharmaceutical industry, to accelerate Pharma 4.0™ transformations. Also known as the Smart Factory, the objective of Pharma 4.0 is to enable organizations involved in the product lifecycle to leverage the full potential of digitalization to provide faster innovations for the benefit of...
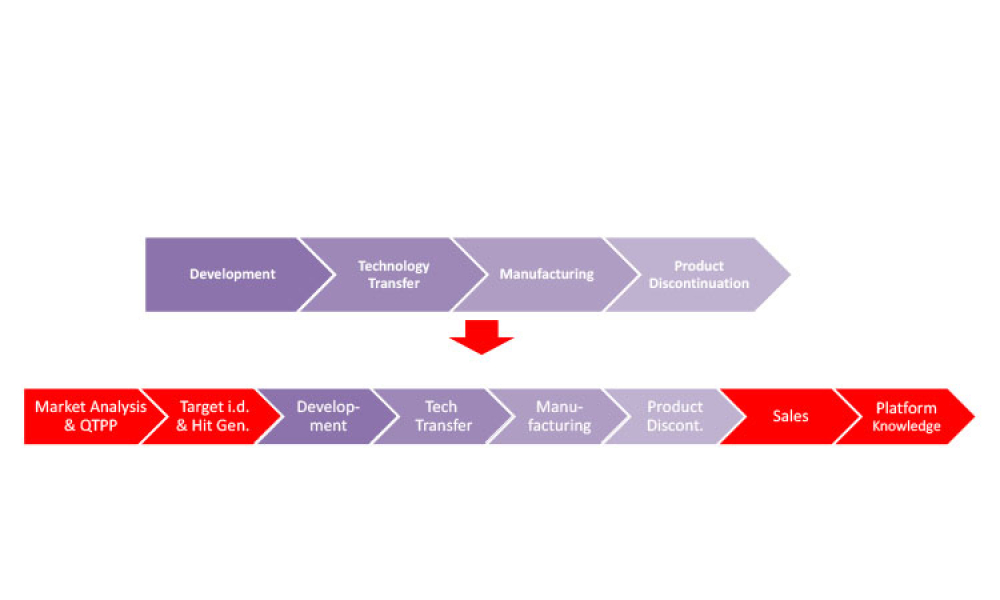
This second of a two-part series explores digital transformation and digitalization in the biopharmaceutical industry with information about how data science enables digitalization along the product life cycle. (Part 1 was published in the March-April 2021 issue of Pharmaceutical Engineering.
Robotic process automation (RPA) software automatically handles manual, repetitive, time-consuming, and highly structured tasks such as data entry and back-office functions. Certain processes specific to the pharmaceutical industry represent strong candidates for RPA implementation, with significant potential savings and the possibility of ensuring compliance.
Industry 4.0 is the recent movement toward intelligent automation technology. In this new era, the integration of modern manufacturing skills and novel information technologies plays an important role on economic competitiveness.
Across every industry today, digitalization is driving the use and value of data to disrupt traditional business models and ways of working. In pharmaceuticals, the promises of Industry 4.0 are expected, and needed, to finally modernize the legacy approaches that have evolved since the 1970s. Validation is an obvious target for digital disruption because of the inefficient, document-heavy...