Data Governance is currently utilized in many areas of business and government including finance, banking, telecommunications, and by the agencies regulating these industries. In pharmaceuticals, digital validation is still at a relatively early stage of the digital transformation journey where data governance is currently managed in silos of data (Industry 3.0) and the industry has of yet to...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Pharmaceutical Engineering® is delighted to announce the finalists for the 2022 Roger F. Sherwood Article of the Year Award. The articles were selected by judges of 36 feature and...
Opening the 2023 ISPE Europe Annual Conference on 8 May, Peter Twomey, Head of Inspections for the European Medicines Agency (EMA), offered a keynote address titled International Cooperation, Harmonization, and Reliance in GMP.
In the beautiful city of Barcelona, Spain, ISPE will host members, vendors, pharmaceutical companies, consulting firms, and design companies for the 2023 ISPE Pharma 4.0™ and Annex 1 Conference on...
Emer Cooke, Executive Director of the European Medicines Agency (EMA), delivered an impactful keynote address at the 2023 ISPE Europe Annual Conference, held 8-10 May , in Amsterdam, The Netherlands. Cooke...
Tracy Jamerson is the Chapter Manager for the ISPE Midwest Chapter. She brings 10+ years of experience to the pharmaceutical industry, currently working as the Office Coordinator at Compli, - a wholly-owned subsidiary of George Butler Associates. Compli is part of the Life Sciences Division. GBA is multi-disciplinary. In this role, she is responsible for a variety of duties, including Project...
The 2023 ISPE Annual Meeting & Expo is just around the corner, taking place at Mandalay Bay in Las Vegas, NV from the 15th to the 18th of October 2023. As a distinguished member of the pharmaceutical...
I have been active in ISPE for many, many years, but when I attended my first ISPE Pharma 4.0™ and Annex 1 Conference in Manchester back in 2019, I was surprised by how few people I knew. I realized that my network within ISPE was very much focussed on Aseptic, Manufacturing, and Quality, and not so much on Digitalization and Pharma 4.0™.
The global pharmaceutical industry has faced unprecedented challenges in recent years, with the COVID-19 pandemic serving as a wake-up call for the vulnerabilities within the pharmaceutical supply chain. As the world strives to emerge from the grip of the pandemic and restore activities reminiscent of pre-pandemic times, uncertainties surrounding supply chain stability persist. The
A one-day seminar titled ICH Q9(R1): The Next Frontier was hosted by the Pharmaceutical Regulatory Science Team (PRST) at the Technological University Dublin (TU Dublin) on 1 June 2023. Professor Anne Greene of TU Dublin and Marty Lipa, Merck & Co., co-chaired the seminar....
The 2023 Europe Hackathon was the largest and most successful one yet. 36 Emerging Leaders from 12 countries descended on Amsterdam, the Netherlands on the weekend preceding the 2023 ISPE Europe Annual Conference to formulate their creative solutions to a real-world pharmaceutical manufacturing challenge.
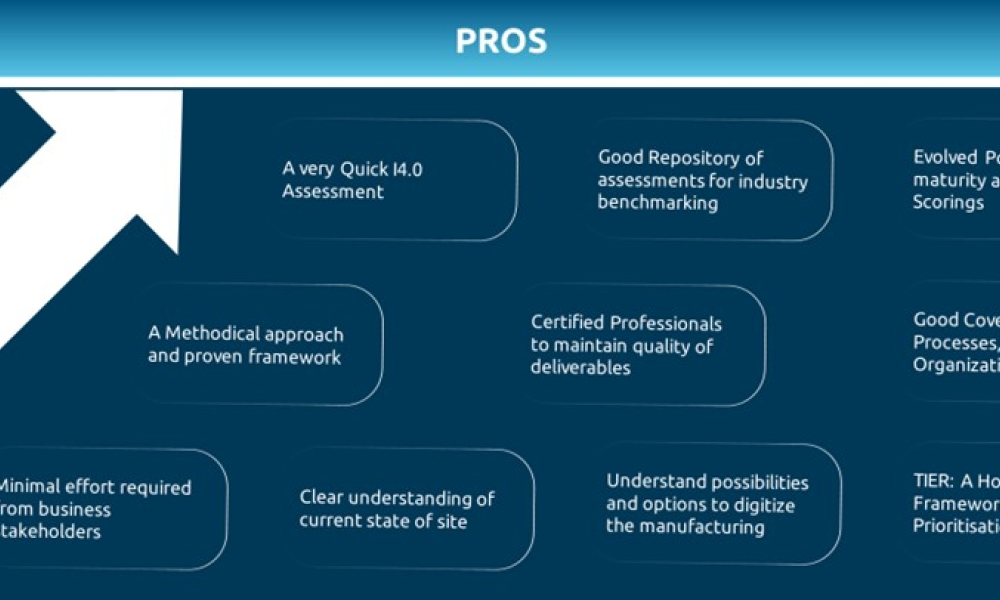
The Smart Industry Readiness Index (SIRI) is a valuable framework that serves as a solid foundation for the Life Sciences 4.0 journey in Pharma, Biopharma, and Medical Devices manufacturing plants. However, it does have its own shortcomings. This blog presents our point of view, covering the strengths and weaknesses of the SIRI framework, and suggests a potential way forward to complement its...
With over 30 years of successful leadership experience in human asset development, building and sustaining operations activity, new project builds, project management, merchandising and new start-up sales/operational fundamentals, Jim Hubbard brings a wealth of experience to ISPE.
An informative and lively regulatory panel discussion was held at the 2023 ISPE Europe Annual Conference in Amsterdam, The Netherlands on 9 May 2023. The session included regulators from the European Medicines...
The upcoming challenges and opportunities of digital transformation were the topic of a panel discussion held on 9 May 2023 at the 2023 ISPE Europe Annual Conference in Amsterdam, The Netherlands.
The 2023 ISPE Annual Meeting & Expo features Manufacturing, Quality Control, and Operational Excellence as one of the key content areas. This comprehensive education program provides attendees with...
ISPE’s Women in Pharma® group advocates for accelerated equality in the pharmaceutical industry and beyond. Through virtual and in-person programming, Women in Pharma provides ISPE members the resources necessary to challenge the status quo and make an impact on both a personal and professional level.
This summer has been, and will continue to be, quite busy with...
Paperless validation – Oxymoron, perhaps? Is there really a truly paperless validation solution out there, one that fits the needs of the validation lifecycle? The ISPE Sub-Committee for Paperless Validation has defined ‘Paperless Validation Systems’ as Paperless solutions enable validation lifecycle deliverables to be generated, approved, and more importantly, testing to be completed without...