Read, Learn, Innovate: Pharmaceutical Engineering® Top 5 Online Articles in May 2021
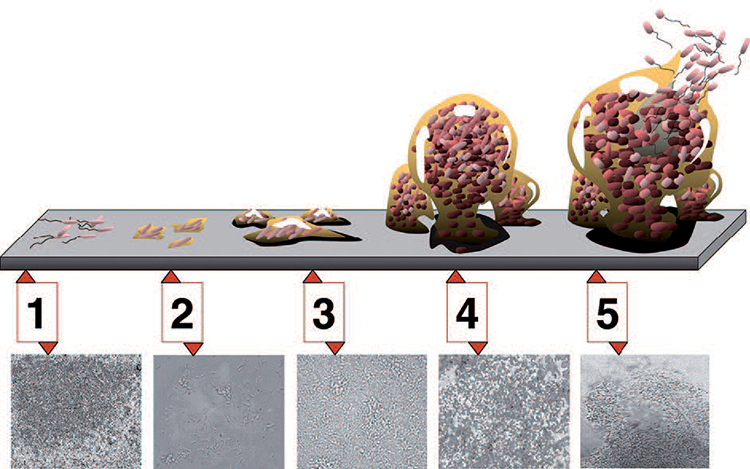
Featured in this edition of the Pharmaceutical Engineering Online Reading Roundup are the most read online articles during May 2021. Learn more about cleanliness classifications for facilities, steam sterilization, GAMP®, and more from what visitors to the PE Online site were reading last month