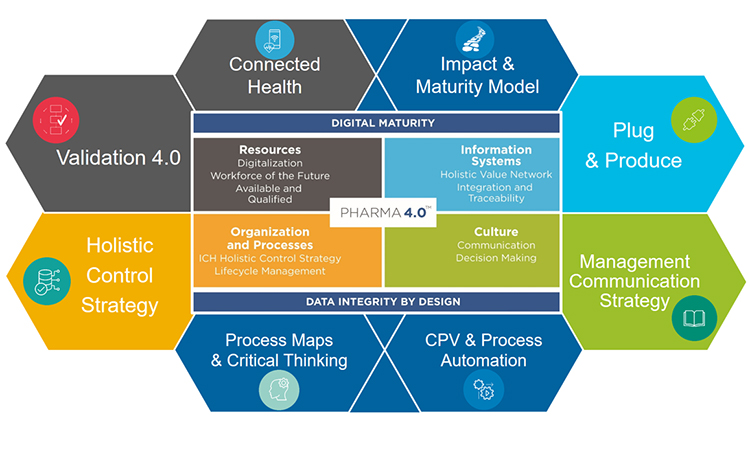
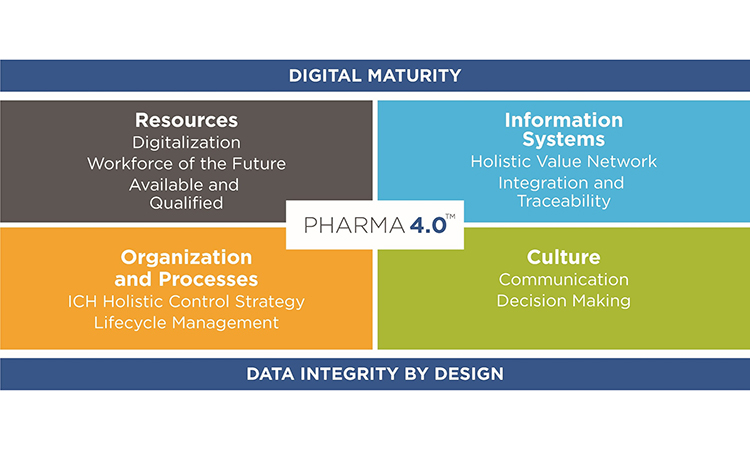
While Pharma 4.0™ has been called a new industrial revolution, its implementation will more likely resemble an evolution in which digitalization and automation meet very complex product portfolios and life cycles. It is therefore important to achieve an accepted understanding of readiness, structural capabilities, and maturity, starting with additional digital enablers and elements added to the ICH Guideline Q10, Pharmaceutical Quality System, along the product lifecycle. It is also important to develop business cases to showcase which automation and digitalization technologies can be applied to pharma and which implications we are facing due to the increasingly complex regulatory challenges in the pharma and biotech industry.
ISPE’s Pharma 4.0™ Special Interest Group (SIG) has developed an operating model to apply the principles of Industry 4.0 to Pharma 4.0™. The group also shares information and education through webinars, articles, the Pharma 4.0 (SIG), and conferences.
On 8-9 December ISPE will be hosting the 2021 ISPE Pharma 4.0™ and Annex 1 Conference in Vienna, Austria. The conference will bring together leading pharmaceutical and biopharmaceutical manufacturers, technology providers, academic scientists, and international regulators to network, share insights, and provide an outlook on the evolving landscape and future of Pharma 4.0™ supported Aseptic Manufacturing.
The conference will provide a leading interdisciplinary platform for all stakeholders of the pharmaceutical chain. This platform will showcase recent innovations and trends, and directly discuss practical challenges and solutions, from a technical, logistical, and regulatory perspective. The impact of upcoming regulations, in particular for Annex 1, will be integrated throughout the conference program and panel discussions. Participants will have the opportunity to engage with industry leaders, regulators, and peers through networking events and the conference program.
CHEManager International, a trade and business newspaper for management in the chemical and pharmaceutical industry, talked to members of ISPE’s Pharma 4.0 Group, industry experts Josef Trapl, Global Head of MSci Innovation, Takeda Pharmaceuticals International, Wolfgang Winter, OpenLab Platform R&D Director, Agilent, Christian Woelbeling, Executive Industry Advisor & Senior Strategic Account Manager, Körber Pharma Software, and Thomas Zimmer, Vice President, European Operations, ISPE, about the idea behind this initiative and the challenges on the way to realize the digital transformation of the pharmaceutical industry.
Read Full Article