Accelerating Biopharmaceutical Virtual FATs in a Pandemic

Heightened awareness, due to the pandemic, of the need for domestic manufacturing capacity has rejuvenated the biopharmaceutical manufacturing industry and resulted in new commissioning projects. However, cross-country/continental travel restrictions and social distancing–based work protocols during the first two years of the pandemic necessitated adopting unique commissioning approaches. Developing standardized factory acceptance test (FAT) execution approaches fit for the current times can allow for consistency across equipment vendors and their biopharmaceutical clients. This article describes pragmatic best practices that would support the momentum for new domestic manufacturing facilities.
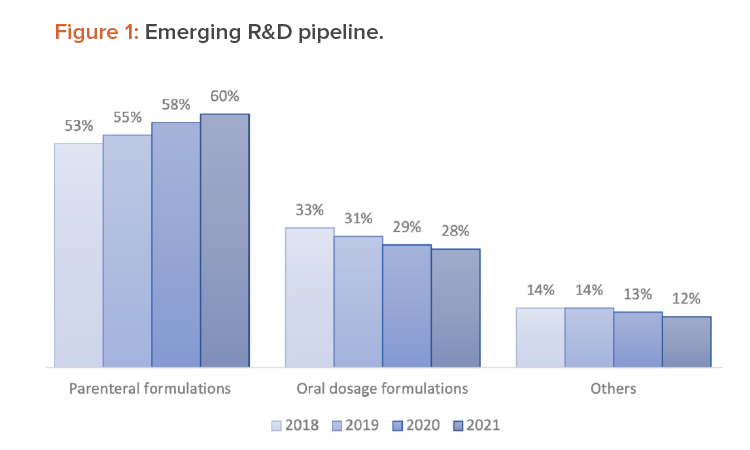
Assessment of the emerging R&D trend from 2018 to 2021 (see Figure 1) indicates a growing tendency to develop new parenteral delivery formulations and a dipping pipeline for oral dosage delivery forms.1
The biopharmaceutical research and development (R&D) sector continues to grow year over year. US firms are the primary investors in biopharmaceutical R&D, with global share of more than 46%.1 The US is also the world’s largest biopharmaceutical market, generating more than 33% of global revenue. Despite a strong R&D sector in the US, outsourcing of formulation development and commercial manufacturing has become an industry norm. This allows innovators to focus on discovering and developing value stream R&D pipelines. But with this competition, it has become standard for commercial development and manufacturing organizations to look for opportunities to lower costs.
One measure devised by innovators and service providers is moving commercial manufacturing activities to countries that offer lower wages and lower indirect costs.2 Identifying this trend as a national security risk, the US Food and Drug Administration introduced a number of effective regulatory policy initiatives to accelerate reshoring and increasing domestic manufacturing capacity.3 The increasing pipeline of parenteral dosage forms, combined with domestic R&D investments and the supply security concerns, provides new renovation and expansion opportunities for domestic vendors to capture biopharmaceutical clients. Awareness of the expectations and availability of the desired tools can enhance virtual FAT execution readiness, in turn meeting the critical biopharmaceutical capital project timelines.
A Regulatory Perspective
In April 2021, the FDA published guidance on remote interactive assessment of manufacturing facilities,4 which was implemented without prior public consultation due to the pandemic. The guidance highlights the regulator’s flexibility in adopting special mechanisms during unusual circumstances. The guidance identifies three phases for a remote interactive evaluation: planning, conducting, and concluding.
Planning
The planning phase considers the type of assessment, the facility risk, and the site’s ability to share live video, documents, and computer screens. The phase identifies the FDA’s and the site’s points of contact and collects background information to effectively coordinate remote assessment.
Conducting
While conducting the interactive assessment, the regulator may request documents, electronic systems visibility, and the use of live-streamed or pre-recorded video to examine the facility or data. The regulator may schedule interviews, evaluate corrective actions, and provide online updates. The regulator expects that the remote connection (such as internet connectivity, image quality, cameras) used is continuous, except for temporary issues that can be resolved in a timely fashion.
Capable technologies are expected to be employed for remote viewing and evaluation of critical operations such as aseptic practices, equipment cleaning, setup, material weighing, dispensing, instrumentation, sampling, and testing. The FDA identified three IT platforms—Microsoft Teams, Zoom, and Adobe Connect—for remote assessments. Some of the reviewed documents may be requested in advance.
The expectation is that the information is provided in electronic format or that access is provided for remote screen sharing so that the documents can be efficiently assessed during a live session. Secure protocols to send documents are provided by the regulator. Where a paper format document is requested, the expectation is to send it to the requestor as a scanned and searchable PDF file.
Concluding
The concluding phase culminates in a closeout meeting with observations (where applicable) where the regulator may determine the need for follow-up surveillance activities. The regulator, as a representative of the agency, can use the remote assessment outcome for regulatory decision-making.
FAT Challenges
As in the case of regulators, biopharmaceutical manufacturers commissioning new facilities face a similar logistical challenge with equipment vendors. Conducting on-site FATs at vendor locations is not always feasible during a pandemic. Design and functional features—such as size, utilities, controls, cleaning, contamination, integrity (data and system), safety, operations, capability, components, quality, installation, drawings, integration, and claims—need to be verified early enough to ensure that the unit meets the organization’s business and GMP needs.
In addition to ensuring they follow CFR section 211.42, the GMPs further mandate verification and adequacy of equipment construction (CFR 211.65); equipment cleaning and maintenance (CFR 211.67); automatic, mechanical, and electronic equipment (CFR 211.68); and filters (CFR 211.72). FAT is, therefore, a critical step in the commissioning phase because it provides documented evidence that a piece of equipment or system has been adequately tested at the equipment manufacturer’s facility and performs to the biopharmaceutical manufacturer’s expectations prior to its delivery.
With the appropriate tools and controls, the goals of FAT can be effectively met despite being virtual in nature.
The interactions during FAT can identify vendor GMP deficiencies so that early measures can be put in place. Typically, the biopharmaceutical company's technical teams are present at vendor sites for the hands-on FAT assessment phase of commissioning: a difficult proposition during a pandemic. The emergence of virtual cross-functional teams—consisting of project managers, project engineers, consultants, contractors, subject matter experts, and vendors—with members across different time zones further complicates the on-site execution of FAT, a critical commissioning stage. Therefore, developing a systematic remote assessment approach modeled in line with the FDA guidance approach described above is ideal.
FAT testing and documentation provide early insight into whether the vendor can meet the biopharmaceutical client’s contractual points and system requirements. The FAT allows for making timely corrections, helps avoid returns, and minimizes component upgrades on final installation. Successful completion of FAT is a precondition to shipment. Involvement with the vendor allows for the biopharmaceutical client’s technical team to understand specifics of the critical system operation, potential challenges, safety hazards, and details such as disassembly/reassembly. A FAT also allows for seamless site acceptance testing (SAT): The initial FAT testing outcome often drives the downstream SAT requirements. FAT test cases, where justified, can also be adopted for qualification.
A FAT has prerequisites such as an in-place vendor quality plan, user requirement specification (URS), functional specifications, and design specifications. As part of the FAT, vendor documentation is verified; this can include, but is not limited to, operation and maintenance manuals, a component list with catalog cut sheets, diagrams, certifications, materials of construction, and inspection records.
The activities part of a FAT can be broadly categorized into review of prerequisites for FAT, verification of supporting documents and inspection records to meet FAT protocol requirements, functional test checks, documentation of corrective measures for identified deficiency findings, and closure and shipment.5,6
Model for a Virtual FAT
Based on practical application, a sterilizer example table (see Table 1) was developed to identify best practices in accomplishing each of the FAT needs through virtual execution. A similar approach can be applied for other systems such as that of complex filling lines and packaging systems. The closure timeline for each FAT phase (planning, conducting, and concluding), document control, and data integrity/governance requirements can be developed as part of the commissioning strategy. The preliminary plan can be agreed upon with the vendor prior to issuing a firm purchase order.
The methods of substantiation during a virtual FAT execution need to be predetermined, in addition to having a preapproved FAT protocol. Where necessary and applicable, the following need to be specified for each FAT task: a combination of live video, recordings, control system screen sharing, and remote access to verify setup parameters; real-time remote monitoring of operating parameters; and contemporaneous documentation. The bio-pharmaceutical client’s team is responsible for the data integrity/governance of the collected evidences. Figures 2–3 provide examples of supporting evidence used when successfully executing FAT in a remote setting.
| FAT Requirements/Tasks | Completed During (Phase) |
Remote Execution/Interactive Tools | Evidence |
|---|---|---|---|
| Review prerequisites | |||
| Vendor approval | Planning |
Compile internally and procure from vendor using a secured Examples of commercially available cloud-based secure |
Electronic PDF document |
| Purchase agreement | |||
| Commissioning plan | |||
| URS | |||
| Functional specifications | |||
| Hardware design specifications | |||
| Software design specifications | |||
| Installation and operating manuals | |||
| Software manual | |||
| Maintenance manual | |||
| Component data sheets | |||
| Component manufacturer information | |||
| FAT protocol | |||
| FAT execution plan | |||
| Participants list | |||
| Verify supporting documents and inspection records meet FAT protocol requirements | |||
| P&ID and general arrangement drawings | Conducting | Vendor to provide the documents in a secured shared folder to review as part of FAT execution. | Electronic PDF document |
| Electrical schematics | |||
| Pressure vessel dossier | |||
| Welding inspection records (welding quality procedures, weld logs, welder certification, visual/boroscopic/ radiographic inspection photos or videos) |
|||
| Material of construction certification and roughness reports | |||
| Test instrument qualification and calibration certificates | |||
| Safety valve certificates | |||
| Declarations | |||
| Controller configuration specification | |||
| Critical instrument calibration certificates | |||
| Roughness reports | |||
| Welding endoscopic check report | |||
| Passivation certificate | |||
| FAT test methods | |||
| Hydraulic pressure test reports | |||
| Slope check reports | |||
| Software qualification package | |||
| Compliance certificates | |||
| FAT Requirements/Tasks | Completed During (Phase) |
Remote Execution/Interactive Tools | Evidence |
|---|---|---|---|
| Perform prefunctional test checks | |||
| Check test instrument calibration and qualification status | Conducting | Vendor to submit the completed documentation in the secured shared folder. Verify prior to start of FAT test cases and document the verification. |
PDF of calibration and qualification records |
| Verify operator safety elements | Vendor to submit to secured shared folder the time/date stamped video recordings. Verify prior to start of FAT test cases and document the verification. |
Live video streaming and recording | |
| Document FAT installation conditions |
Vendor to submit the completed documentation to the
|
Live video streaming and recording
|
|
| Verify access to filter, sample valves, gauges, and perimeter | |||
| Verify all piping components are correctly fitted and labeled | |||
| Check piping insulations | |||
| Check utility loops, connections, and readings | |||
| Perform dimensional and surface checks: chamber, doors, and panels | |||
| Verify instrumentation displays, e.g., manometer | |||
| Check nameplates/U stamps | |||
| Check components | |||
| Verify system dimensions | |||
| Check doors and panels | |||
| Confirm electrical options | |||
| Check electrical cabinets with markings and inspection certificate | |||
| Check that wiring diagram and as-built matches | |||
| Confirm hydraulic options | |||
| Confirm accessories options | |||
| Confirm cabinet options | |||
| Verify rails, load, and height | |||
| Check internal and external trolley specs | |||
| Check P&ID, general arrangement, and electrical schematic diagram vs. as-built | |||
| Perform FAT functional tests | |||
| Check internal and external trolley braking system | Conducting | Vendor to submit the completed documentation to the secured shared folder along with time/date stamped video recordings. |
Live video streaming and recording PDF of the executed test |
| Verify loading and unloading | |||
| Check chamber vacuum leak rate test | |||
| Conduct safety systems challenges: emergency buttons, level sensors, start, interlock, opening/closing |
Vendor to submit the completed documentation to the secured shared folder along with time/date stamped video and screen recordings.
|
Live control system screen sharing to
|
|
| Cycle functional tests to verify cycles refl ect the setup parameters and no alarms are activated: vacuum leak rate test, pressure leak rate test, F0/time-based sterilization cycles, chamber air brake filter sterilization cycles |
|||
| Perform software test cases | |||
| Confirm empty chamber temperature mapping and loaded chamber temperature mapping (terminal sterilizer) |
|||
| FAT Requirements/Tasks | Completed During (Phase) |
Remote Execution/Interactive Tools | Evidence |
|---|---|---|---|
| Address identified deficiencies, findings, and corrective measures | |||
| Revisit test failures needing redesign and retesting | Concluding | Vendor to submit the completed documentation to the secured shared folder. |
PDF documents |
| Perform tests that could not be executed at FAT location | |||
| Make modifications to meet URS requirements | |||
| Add sensors and monitoring devices | |||
| Complete other minor punch list items before shipment | |||
| Fix documentation errors | Revised approved PDF documents | ||
| Identify new design, develop internal quality test, and reperform FAT |
Planning > Conducting > Concluding |
Not applicable | New FAT |
| Finalize retest requirements after corrective actions | Conducting > Concluding |
Vendor to submit the completed documentation to the secured shared folder along with time/date stamped video and screen recordings (as required). | Approved retest PDF documents |
| Confirm closure and shipment details | |||
| Conduct closure session | Concluding | Client and vendor to record the event and store in a secured folder. |
Live video streaming and recording |
| Verify closure of punch list item | Vendor to submit the completed documentation to the secured shared folder. |
Electronic PDF document | |
| Confi rm final shipment packaging configuration | |||
| Verify shipping mode/conditions/storage impact | |||
| Verify adequacy of packaging components | |||
| Approve FAT and shipment | Vendor to share the approved document through the secured shared folder. Example of a commercially available 21 CFR Part 11 compliant document management software: MasterControl |
Electronic PDF document | |
Notes: 1. Once reviewed, the final approved documents and evidences are available in the biopharmaceutical client’s qualified document management system. 2. Records, videos, screen recordings, and interviews documented/shared should be attributable, legible, contemporaneous, original, and accurate (ALCOA) and adhere to industry standards for data integrity.

Emerging Technologies
Deploying new virtual technologies can enhance remote FATs. Digital twin is a technology that can simulate testing of multiple variants while reducing any material waste. Digital twin technology can permit virtual commissioning,7 has been helpful in exposing defects early, and has enhanced engineering efficiency by almost 30%.8 Although some augmented reality and virtual reality hardware are still evolving for use in cleanrooms, they can be effectively used in unclassified areas such as an equipment vendor’s FAT test floors. The technology can be used to provide assisted remote training to a biopharmaceutical client’s technical teams to familiarize them with equipment components, teach them troubleshooting techniques, and demonstrate equipment operation prior to taking part in FAT execution. Pharma 4.0™ machine learning and artificial intelligence sensors are being integrated into equipment so that process optimization and monitoring can be achieved once in use. And the same sensors and process modeling software can play a key role while remotely assessing equipment drift during FAT trials.

Conclusion
The approaches discussed in this article can be applied to downstream commissioning steps such as SAT and qualification. One of the goals of FAT is to ensure that the equipment is evaluated at the vendor site prior to shipment and delivery. Completion of the predefined test cases corroborates compliance with URS/functional specifications. Although virtual solutions exists for both document assessments (such as certificates and P&ID) and functional tests, organizations may consider FAT for complex systems. Higher levels of interaction with vendor subject matter experts and the opportunity to have early hands-on training during an on-site visit can be invaluable. Therefore, it is important that during the virtual execution, the vendor provides access to their key technical personnel, gives sufficient opportunity to discuss details, and clarifies features.
A well-conducted virtual FAT eliminates traveling during a pandemic while saving on the associated expenses (capital expenditures, or CAPEX). With the appropriate tools and controls, the goals of FAT can be effectively met despite being virtual in nature. The several FAT executions that we conducted using the model discussed here have further established that a well-planned application of virtual tools and data governance policies can be comparable to an in-person execution. The FAT report becomes the basis of determining the on-site SAT protocol requirements on receipt and the extent of the SAT/qualification test executions.
The checks and verifications performed during FAT can be used to support qualification activities, and they do not need to be repeated, provided there is risk-based justification that the functionality will not be affected by any subsequent activities prior to acceptance and release.5 The additional practices discussed can prove advantageous for biopharmaceutical organizations as they immediately convert the otherwise tacit equipment knowledge into codified explicit knowledge for downstream use. A key goal while developing a virtual FAT program should be to preserve conformity with internal procedures, industry standards, and regulatory guidelines.







