ISPE Briefs: New Guidance Document Portal Offers Enhanced Features
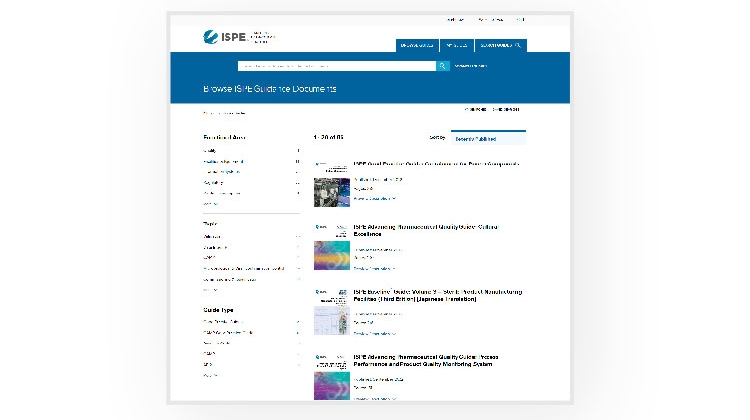
In August, ISPE unveiled a new Guidance Documents Portal that provides a significantly improved online user experience for ISPE’s full library of Guidance Documents.
Developed in partnership with Wiley, a global leader in scientific publishing, the new portal enables ISPE to improve the effectiveness and impact of ISPE Guidance Documents by leveraging Wiley’s global reach, publishing experience, and advanced technologies to help ISPE better achieve our mission. ISPE and Wiley will work together to develop continual advancements.
Benefits of the new portal include:
- Efficiency and ease: Navigate with a fresh, intuitive design for improved user experience.
- Comprehensive search: Access the complete ISPE Guidance Document library effortlessly with enhanced search.
- Adaptable reading: Easily dive into content with an integrated e-reader.
- Seamless mobile access: Experience excellence on any device with responsive design.
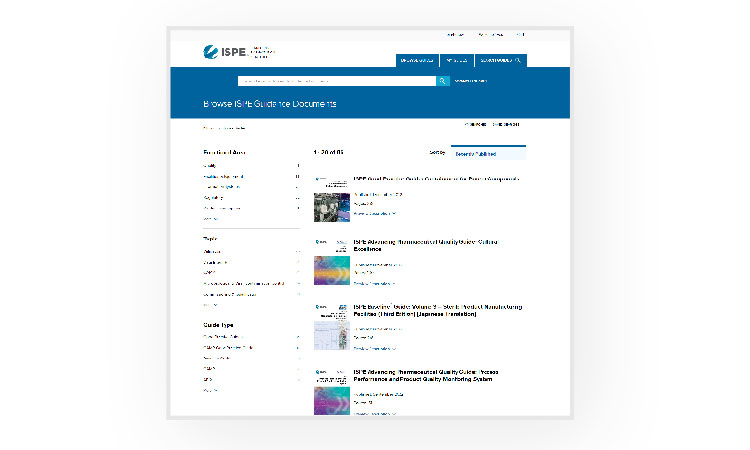 To access the Guidance Document Portal, log in to the ISPE.org website and navigate to the Guidance Document Portal. Purchased guides and member benefit guides can be accessed in the My Guides section. Please note that the select ISPE Good Practice Guides included with membership are available for view-only access in the e-reader.
To access the Guidance Document Portal, log in to the ISPE.org website and navigate to the Guidance Document Portal. Purchased guides and member benefit guides can be accessed in the My Guides section. Please note that the select ISPE Good Practice Guides included with membership are available for view-only access in the e-reader.
ISPE Unveils First-of-Its-Kind Guidance Document and Launches New Community of Practice on Pharmaceutical Compounding

ISPE is proud to introduce two significant initiatives aimed at benefiting the field of pharmaceutical compounding—the release of the ISPE Guide: 503B Compounding – Regulatory Basis and Industry Good Practices for Outsourcing Facilities and the launch of the ISPE Community of Practice (CoP) on Pharmaceutical Compounding.
Regulated by a diverse range of state boards with their own expectations, compounding pharmacies in the United States have historically faced challenges due to the absence of a nationally accepted approach to compliance. The introduction of Sections 503A and 503B to the Food, Drug, and Cosmetic (FD&C) Act in 2013 set regulatory expectations for compounding pharmacies and Current Good Manufacturing Practice (cGMP) outsourcing facilities. In addition, the FDA issued a draft Guidance for Industry in 2020 on the agency’s current thinking on regulatory expectations for 503B outsourcing facilities.
ISPE Guide: 503B Compounding – Regulatory Basis and Industry Good Practices for Outsourcing Facilities
In response to industry demands and regulatory complexities, ISPE developed this comprehensive guide to serve as an invaluable resource for 503B outsourcing facilities, aiding them in understanding the regulatory requirements outlined in the FD&C Act Section 503B.
The guide combines FDA regulations and recommendations with pharmaceutical industry standards, providing a go-to document for 503B facilities of all sizes. Various aspects of the compounding process are covered, from the importance of cGMPs to establishing a quality system (including qualifying suppliers and vendors), receipt of raw materials/active ingredients, and shipping of finished drug products.
The guide also provides recommendations for facility and equipment design, drawing from aseptic manufacturing practices and scaled to meet the needs of 503B facilities, and presents industry best practices for aseptic manufacturing, emphasizing personnel training and qualification.
We believe these initiatives will play a pivotal role in shaping the future of pharmaceutical compounding, ultimately benefitting patients and communities worldwide.
The guide addresses microbiological and analytical testing, including verifying the suitability of compendial methods and validating non-compendial methods. It covers beyond-use dating, offering essential insights into limited stability testing along with stability best practices. A dedicated chapter on preparing for regulatory inspections provides facilities with a valuable resource for this key aspect in ensuring compliance.
ISPE CoP on Pharmaceutical Compounding
The ISPE Pharmaceutical Compounding CoP seeks to foster innovation to improve the practice of pharmaceutical compounding, and to disseminate ideas, knowledge, and best practices through generation of ISPE content, including guidance documents, Pharmaceutical Engineering® magazine articles, webinars, blog posts, conference presentations, and training materials.
The new CoP will provide a venue for industry and regulatory informal interactions to drive practical and effective design and operational practices while addressing regulatory expectations and providing solid scientific justification for practices accepted by industry and regulators alike.
Individuals interested in being considered for participation on the newly forming Steering Committee to lead the ISPE Pharmaceutical Compounding CoP are urged to email ISPE at communities@ispe.org
“ISPE is proud to provide critical guidance to the pharmaceutical compounding industry with the release of the ISPE Guide: 503B Compounding – Regulatory Basis and Industry Good Practices for Outsourcing Facilities and the launch of the ISPE Pharmaceutical Compounding CoP,” said Tom Hartman, President & CEO, ISPE.
“These comprehensive resources signal a transformative step toward fostering collaboration, innovation, and excellence among professionals in this vital sector, and underscore our commitment to advancing knowledge and enhancing compliance within the industry. We believe these initiatives will play a pivotal role in shaping the future of pharmaceutical compounding, ultimately benefitting patients and communities worldwide.”
ISPE Pharmaceutical Engineering® “Special Report: COVID-19” Honored with 2023 APEX Award

ISPE has been honored with a 2023 APEX Grand Award for Public Health Concerns for the “Special Report: COVID-19.”
This award included two articles, “Pandemic Progress: Industry’s Journey from 2020 to Today” and “Operation Warp Speed: A View From the Inside.” Both articles were published in the May/June 2022 issue of Pharmaceutical Engineering®. This marks the fourth consecutive year that Pharmaceutical Engineering® has received an APEX Award.
The APEX Awards commend excellence in writing, editing, and graphics across a wide range of communications in nonprofit and for-profit publishing and communications organizations. Awards of Excellence recognize exceptional entries in 100 subcategories, and Grand Awards honor outstanding work in 14 major categories.
“Pandemic Progress: Industry’s Journey from 2020 to Today” explores the remarkable evolution of the healthcare and pharmaceutical industry’s response to the COVID-19 pandemic, highlighting the rapid development of vaccines, collaborative efforts among competitors, and advancements in understanding the virus. The article was authored by ISPE member, Wendy Haines, PhD, DABT, CQA, Director of Toxicology & Technical Services, PharmEng Technology.
“Operation Warp Speed: A View From the Inside” provides an inside look at the work done by Operation Warp Speed, a US government initiative supporting the accelerated development and supply of vaccines and therapeutics across the United States to address the threat posed by COVID-19. The article was written by Carlo de Notaristefani, PhD, Consultant, who assumed the role of lead advisor for Manufacturing and Supply Chain at Operation Warp Speed/Federal COVID Response in May 2020.
Meet the ISPE Staff: Victoria Bucci

In each issue of Pharmaceutical Engineering®, we introduce a member of the ISPE staff who provides ISPE members with key information and services. Meet Victoria Bucci, Digital Marketing Manager on the Marketing team.
Tell us about your role at ISPE: What do you do each day?
Day-to-day I manage paid advertising efforts, social media accounts, and curate ISPE content for the SmartBrief newsletters.
What do you love about your job?
I love the wide variety of content available to pull from. Not only do we have regular blog articles and PE magazine editions, but each team also has something for me to help promote. This gives me a chance to test new paid advertising strategies, keep our social media channels active, and share valuable updates and information with our SmartBrief subscribers.
What do you like to do when you are not at work?
Outside of work, I spend a lot of time camping with my husband and our friends. We enjoy getting away for a short weekend of hiking, kayaking, and sitting by a fire. We also love going to comedy shows. We have five planned so far for the rest of the year.




