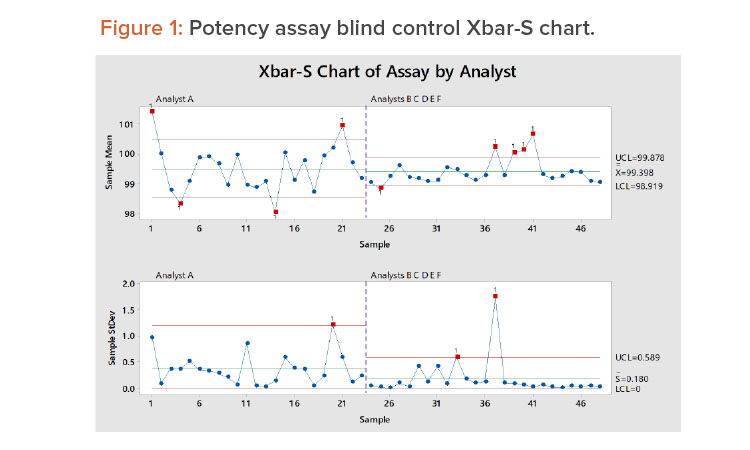
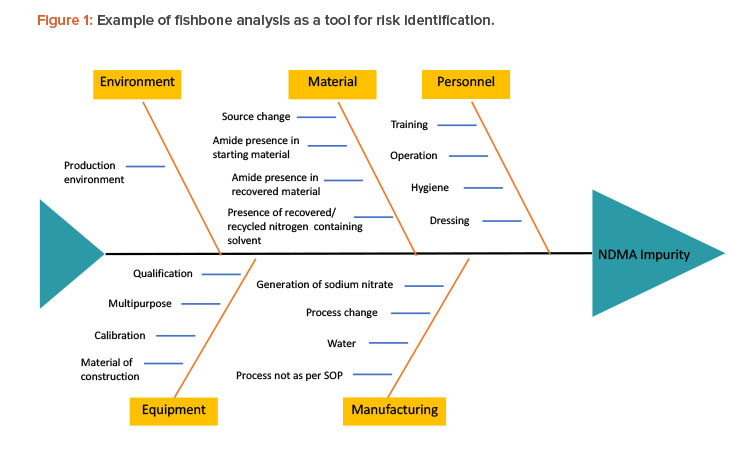
Read, Learn, Innovate: Quality Topics in Pharmaceutical Engineering®
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are recent technical articles about quality, including global commissioning, qualification, and validation; data for verification; and addressing N-nitrosodimethylamine (NDMA) impurities.