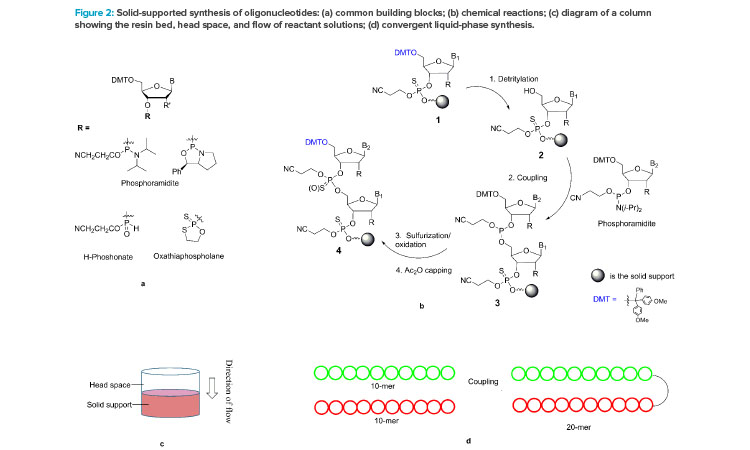
Read, Learn, Innovate: Pharmaceutical Engineering® Holiday Reading Edition
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are some great reading selections for the holidays! This year’s holiday topic: biopharma, cell and gene therapies, and ATMPs. That topic is the theme for the November-December 2021 issue , and here are some earlier articles on these important and timely topics.