PIC/S in Latin America: Harmonization of cGMP Procedures

This article offers an overview of the benefits of Pharmaceutical Inspection Co-operation Scheme (PIC/S) membership for regulatory authorities and industry. It also highlights Latin American regulators’ current perspectives on PIC/S membership to increase awareness and encourage open dialogue about harmonization, recognition agreements, and potential increase for export facilitation, all which will help increase the access of high-quality medicines to patients. Given that only three countries are PIC/S members in the Latin America region, this represents a huge opportunity for cGMP inspection.
In 1995, the PIC/S was established as an extension to the Pharmaceutical Inspection Convention (PIC) of 1970 as a non-binding, informal cooperative arrangement between regulatory authorities in the field of current good manufacturing practices (cGMP) of medicinal products for human or veterinary use.1
PIC/S aims to harmonize inspection procedures worldwide by developing common standards in the field of cGMP and by providing training opportunities to inspectors. It also aims to facilitate cooperation and networking between regional and international organizations and competent authorities, thus increasing mutual confidence. This is reflected in the PIC/S mission statement: “To lead the international development, implementation, and maintenance of harmonized cGMP standards and quality systems of inspectorates in the field of medicinal products.”1
The fact that PIC/S is non-binding allows participating authorities to cooperate and share information informally while keeping complete control over imported medicinal products. This was noted as a key advantage over a mutual recognition procedure by Margaret Hamberg, PhD, former US Food and Drug Administration (FDA) Commissioner.2 PIC/S is open to any authority having a comparable cGMP inspection system.
| Applicants (Up to 6 Years) | Pre-Applicants and Former Pre-Applicants (Gap Analysis Only) | Interested |
|---|---|---|
| Armenia | Azerbaijan | India |
| Bulgaria | China | Bangladesh |
| Russian Federation | Sri Lanka | |
| Jordan | Vietnam | |
| Saudi Arabia | Philippines |
PIC/S in Latin America
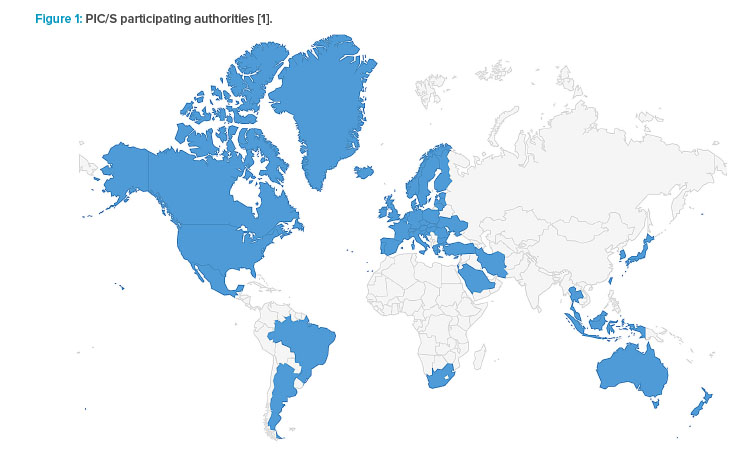
As of 1 July 2023, PIC/S comprises 56 participating authorities from all over the world—Europe, Africa, America, Asia, and Australia—as shown in Figure 1. Latin America comprises 33 countries, with the Caribbean Islands. However, of these, only three countries are members of PIC/S: Argentina, through the Argentina Health Authority, National Administration of Drugs, Food and Medical Technology (ANMAT); Mexico, through the Federal Commission for Protection against Health Risks (COFEPRIS); and Brazil, through the Brazilian Health Regulatory Agency (ANVISA).
As indicated in Table 1, the candidates for PIC/S membership are growing; nevertheless, there are no new candidates from Latin America. There is a great opportunity for cGMP inspection in the Latin America region seeing that only three countries are PIC/S members.
Membership to PIC/S
An essential prerequisite to become a member of PIC/S is that the applicant authority must have a fully functional quality management system (QMS). This is also the main difference from a cGMP perspective between PIC/S and the World Health Organization (WHO). The WHO includes countries with developing quality systems and cGMP programs and hence has a broad list of countries. The PIC/S requires the QMS to cover the systems and procedures of the inspectorate: cGMP inspection procedures, manufacturer licensing procedures, document control procedures, complaint and recall handling procedures, a program for the training of inspectors, code of ethics for inspectors, and more.3
Before an authority is accepted by PIC/S, a detailed assessment is undertaken to determine whether the authority can apply an inspection system comparable to that of current PIC/S authorities. This assessment, called the accession process, is a 12-step process. In theory, this process can be completed in 18 months, but for most applicants it takes three to five years.
The accession process involves an examination of the authority’s cGMP inspection and licensing system (or equivalent), quality system, legislative requirements, inspector training, etc. It is followed by a visit by a PIC/S delegation to observe inspectors carrying out routine cGMP inspections and includes a visit to the government analytical laboratories. During the process, changes and improvements might be recommended by the PIC/S Committee and where necessary, follow-up visits are undertaken to verify the suitability of corrective actions.4
A PIC/S pre-accession process was introduced to better prepare potentially interested authorities for PIC/S accession. The main advantage of the pre-accession procedure consists in the carrying out of a pre-assessment by an auditor. This includes a gap analysis conducted by the applicant and monitoring by PIC/S-appointed rapporteur(s) to determine areas of noncompliance with PIC/S requirements. The pre-accession process also enables a regulator to determine whether they are ready to engage in a full application.
PIC/S Activities
The main activities of PIC/S are:
- Experience: Appropriate training for new and established members of the cGMP inspectorate
- Seminars: PIC/S meetings and training seminars are not open to industry
- Joint Visits program and coached inspectors: Inspectors from three different countries are teamed up to observe cGMP inspections to compare inspection procedures and techniques and to harmonize cGMP interpretation
- Expert circles: Discuss and exchange information on specific technical areas of cGMP, develop draft guidance documents, and provide training opportunities in their field of expertise
- Guidance documents: Development and promotion of harmonized cGMP standards and guidance documents
- Exchange of information: This scheme relies on the exchange of information on cGMP inspections on a purely voluntary basis; there is no obligation whatsoever to accept inspection results4
The authors prepared a survey with the goal of increasing awareness of PIC/S in other Latin America countries, to encourage an open dialogue about harmonization and reliance, and to highlight the benefits for the Latin America region.
PIC/S Benefits
The benefits for members include:
- Access to an approved reference for pharmaceuticals manufacturing facilities inspection
- Enhanced efficiency and quality of manufacturing practices of local factories
- Harmonized and standardized inspection procedures at the level of Member States in GMP
- A rapid alert system at the level of Member States
- Efficiency of the inspectors and training opportunities
- Recognition of exporters that comply with international cGMP standards
- Community adoption of international cGMP standards
- A more effective use of inspection resources through the voluntary sharing of cGMP inspection reports
Additional benefits of PIC/S membership for cGMP regulatory authorities include:
- An internationally recognized system of cGMP controls, including a quality system for the cGMP Inspectorate
- Inspection reliance: the 56 member authorities of PIC/S rely on each other’s inspections and inspection reports, as they help avoid duplicate and same scope inspections
- Training opportunities for inspectors, including annual training seminars of specific topics, expert circle on technical topics, annual training seminars for new inspectors, joint inspector training, coached inspections, and access to the PIC/S Inspectorates’ Academy for online training
- Opportunities for networking and information sharing among all inspectors of the PIC/S member authorities, particularly during the training events
- The opportunity to participate in the development of internationally recognized cGMP guides and guideline documents
- Involvement in a non-political forum for cGMP regulators
History of PIC/S in Latin America
Argentina, ANMAT
ANMAT acceded to PIC/S in January 2008,5 after a meticulous evaluation process that determined that the body has an inspection system equivalent to that of the high surveillance regulatory authorities belonging to PIC/S.
In line with the Joint Reassessment Programme, ANMAT was reevaluated by PIC/S in November 2018. Members of the PIC/S audit team reassessed the standards of GMP and quality systems of ANMAT’s inspectorate. The delegation—made up of officials from the regulatory authorities of France, the Netherlands, Spain, and Israel—met with professionals from the General Coordination of the Inspectorate and the National Institute of Medicines to analyze the regulatory systems and inspection procedures for drug plants. The evaluation included a visit to various pharmaceutical plants in the country.6
In April 2019, at the 47th PIC/S Meeting, the Compliance Subcommittee (SCC) informed ANMAT that they complied with the good practices standards and quality systems of their inspections for medicines. In doing so, ANMAT also successfully obtained full membership of the PIC/S regime.7
Mexico, COFEPRIS
COFEPRIS acceded to PIC/S in January 2018.5 The process followed by COFEPRIS to enter PIC/S corresponds to the relevance of being part of this international cooperation initiative that facilitates, strengthens, and maintains mutual trust among its members in the field of inspections of GMP for pharmaceutical products.
COFEPRIS assumed the commitment to align the regulatory processes related to the standards established by PIC/S. This was done through intense institutional work for the development, implementation, and maintenance of actions that would allow the agency to effectively comply with international guidelines. As a result of this process, regulatory capacities in drug inspections have been strengthened with a view to optimize decision-making and the strategic development of inspections carried out by COFEPRIS.
In the context of increasing globalization of the pharmaceutical industry and advances in health sciences, it is essential to have a robust regulatory system. This system should ensure the supply of quality pharmaceutical products that are safe, effective, and economically accessible to the population.
The entry of COFEPRIS into PIC/S recognized the agency’s implementation of health regulation tasks with the following main benefits:8 international harmonization of cGMP allowing participation in the development/update of international guidelines; training opportunities (e.g., seminars, joint inspection visits); the exchange of information and fast alert system among participating agencies; and the facilitation of the negotiation of instruments for recognition of cGMP certification between the member agencies.
Brazil, ANVISA
In 2014, ANVISA formalized its interest in becoming a member of PIC/S. This meant that the agency was willing to start efforts to modernize its regulatory framework, invest in the qualification of inspectors working in the National Drug Inspection System, and harmonize inspection procedures for GMP in all Brazilian states.
The decisive year to boost the process of joining PIC/S was 2019. In that year, director Fernando Mendes and deputy director Meiruze Freitas adopted the process of joining ANVISA to PIC/S as a priority project. A major effort to modernize regulatory instruments and work processes was therefore initiated.
In 2019, the regulatory frameworks related to GMP for medicines and public health laboratories were widely discussed and duly updated. The audit work of state and municipal health surveillance was also intensified. In October 2019, the country received PIC/S inspectors from members of health agencies in the UK, Portugal, Malta, and Hong Kong (who audited ANVISA), the State Health Surveillance of Minas Gerais, and the Municipal Health Surveillance of São Paulo.
Additionally, the Ezequiel Dias Foundation (Funed) in Minas Gerais and private pharmaceutical laboratories were also audited. All this took place for the certification, by foreign health authorities, to ensure that the Brazilian inspection process is equivalent to that of the other countries that are part of PIC/S.
The decision for ANVISA’s PIC/S membership was expected for the first meeting of the PIC/S Committee of 2020, held annually in April. However, due to the pandemic, the decision was postponed until a committee meeting was held on 15 October 2020. On 30 November 2020, ANVISA was formally notified of the approval of its membership. Membership began in January 2021 and ANVISA become the 54th member of PIC/S.
Technical Cooperation in cGMP
Reliance may take many forms, including full recognition of an inspection decision of a reference national regulatory agency (NRA). This allows for the use of minimal documentation to make a subsequent decision by another PIC/S member. Members may also use full, unredacted assessment reports and/or additional information to make an independent decision. The extent of reliance applied, and documentation required, is up to each national health authority agency to determine in line with their capacity capability and legal frameworks.9 Latin America PIC/S members are taking steps toward formalizing regulatory cGMP inspection cooperation agreements in the region.
To that end, ANVISA and ANMAT agreed to exchange inspection certificates issued by both agencies with a view to issuing the cGMP certificates. This was done within the framework of MERCOSUR. MERCOSUR, the Southern Common Market, is a regional integration process, initially established by Argentina, Brazil, Paraguay, and Uruguay, and subsequently joined by Venezuela and Bolivia.
This cooperation agreement was signed in 2006, in the city of São Paulo, where both agencies also agreed that the cGMP certificates issued by them must be related to the historical data of the companies and products, in accordance with national regulation. The exchange of an inspection report is currently still required for each agency to conduct their local verification.10
Recently, during the meeting of the national regulatory authorities of regional reference of the Pan American Health Organization (NRAr/PAHO) in July 2023, the alternatives to strengthening regulatory convergence and reliance in the region were discussed. As a concrete example, officials from Mexico (COFEPRIS) and Brazil (ANVISA) announced that they are working toward regulatory convergence and recognition of inspections and good manufacturing practice certification through PIC/S.
This step indicates an expansion of scope of existing cooperation agreement in place between the agencies. When implemented, it would help realize benefits for both countries, under the auspices of PIC/S, around joint inspection visits to standardize the surveillance processes while ensuring safe and effective health supplies are available in both countries. To advance in this direction would require the confirmation of legal frameworks.11, 12
Perspectives on PIC/S Membership: Survey Results
The authors prepared a survey to obtain the current perspectives of the Latin America regulators on PIC/S membership. This was done with the goal of increasing awareness of PIC/S in other Latin America countries, to encourage an open dialogue about harmonization and reliance, and to highlight the benefits for the Latin America region. Key learnings from the survey responses provided by ANVISA are presented in the following section.
Application Process
Pre-accession process
The pre-accession process provides an effective tool for understanding the audit criteria. Through self-assessment, each agency can identify its gaps, understand its current status in relation to PIC/S requirements, and prepare the necessary action plans for the accession process.
Accession process
PIC/S auditors are trained and perform their functions within what is determined and required by the PIC/S audit checklist. Applicants with a positive pre-accession process, and those that have made the necessary improvements, will not have difficulties in relation to the expectations from PIC/S.
Advice for new applicants
Dig deeper into the pre-accession process. Be sure to thoroughly understand each criterion set out in the PIC/S questionnaire and audit checklist during this step. The audit checklist interpretation guide provided by PIC/S13 is a useful tool for adjusting expectations between the auditee and the auditor.
Member Benefits: Experience for Countries
As a pre-applicant, countries already have partial access to training events, networking, harmonization, and information exchange. With the accession, countries have access to the PIC/S Inspectorates’ Academy, with its vast technical bibliography and training courses available online. The pre-accession process itself leads to harmonization to meet the audit criteria according to PIC/S expectations.
Benefits for Other Countries in the Region: Reliance
Some PIC/S member countries rely on ANVISA inspection reports. Each authority has autonomy and a strategy for dealing with reports issued by partner authorities. ANVISA accepted reports issued by all health authorities that are members of PIC/S during the COVID-19 pandemic (contingency and exceptional measure). The experience was positive, with no increased public health risk. The experience triggered ANVISA to accelerate actions in relation to the creation of a mutual recognition agreement.
Industry Perspectives
There are also related benefits to local industries within Latin America when their relevant regulatory authority becomes a member of PIC/S. These benefits may include the following:1
- Reduced duplication of inspections, especially since the introduction of the PIC/S Inspection Reliance Initiative
- Export facilitation (including export to non-PIC/S countries)
- cGMP control of imported medicines (a level playing field for local industry)
- Improved competitive standing internationally
- Enhanced market access
- Enhanced reputation of local industry
- Transparent inspection standards
- Consistency of cGMP inspections
- Reliable quality medicines available locally and internationally
When a regulatory authority becomes a member of PIC/S, it shows commitment to reliance and harmonization efforts, optimizing the use of inspection resources and increasing confidence and the reputation of the local industry while strengthening the reliable quality of medicines available locally and internationally.
The “Annual Regulatory GMP/GDP Inspection Survey 2022 Data” published by European Federation of Pharmaceutical Industries and Associations (EFPIA) confirms the PIC/S benefits to industry.14 The survey was conducted to monitor trends and new focus areas while promoting the use of inspection resources to materialize the benefits of PIC/S membership. In summary, the survey shows that inspectors are back at manufacturing sites with numbers similar to 2019, after a reduction observed during 2020–2021 influenced by the COVID-19 pandemic.
Additionally, a much higher on-site presence for domestic inspections (about 95%) was found compared to foreign inspections (50%). The survey results demonstrated that routine domestic inspections performed by trusted inspectorates (such as PIC/S member states) are most efficient and demonstrate control of the QMS process. The survey also provided visibility to the inspection practice and the varying degree of acceptance for using electronic files, and requests from industry for regulators to continue cGMP/GDP inspections based on regulatory guidance rather than for-profit standards, which are developed by experts and may have conflict of interest.
A More Effective Use of Resources
PIC/S membership allows a more effective use of inspection resources. The following inspection processes are encouraged for an efficient management of inspection workload: recognition of inspection reports; adoption of flexible inspection modes (on-site, remote, or hybrid); and a risk-based approach to adapt the scope, length, and frequency of inspections.
The establishment of a legal framework to allow acceptance for inspection from PIC/S participating authorities—along with the continuous skill-building and exchange of information—can play an important role in accelerating manufacturing sites’ cGMP certifications. This will also help accelerate access to important medicines to patients.
PIC/S Improvements
Although limited, some positive improvements have been made between PIC/S participating authorities in the Latin America region. Of note is the long-lasting cooperation agreement between ANVISA and ANMAT. This was established in 2006, prior to either agency becoming a PIC/S member, to exchange inspection certificates issued by both agencies. Most recently, on 11 July 2023, the heads of COFEPRIS and ANVISA pledged to design a work plan for regulatory convergence, the recognition of inspection visit reports, and cGMP certificates and to harmonize inspection procedures in both countries.10, 11
The ANVISA-ANMAT collaboration agreement, and potentially the future ANVISA-COFEPRIS collaboration agreement, mark an important step toward the adoption of reliance procedures for regulatory inspections in the Latin America region. Further benefits could be achieved by leveraging full or partial inspection reliance, and ultimately mutual recognition agreements between authorities. Advancing in this direction would make the best use of available resources and expertise, preventing duplication of regulatory assessments. It would also improve and expedite the regulatory inspections while enabling access of quality-assured, effective, and safe medical products to patients.
Conclusion
PIC/S offers a variety of advantages for both participating authorities and industry. However, the full benefits have not yet been achieved in the Latin America region. Only 3 out of the 33 countries are PIC/S members, which represents an excellent opportunity for the non-PIC/S participating authorities in the region. Furthermore, the implementation of formal inspection cooperation agreements and mutual recognition agreements in the region are in the early stages, and there is opportunity to develop and shape this sphere.
About the Authors
Acknowledgements
The authors would like to thank Ana Carolina Moreira Marino Araujo, Ronaldo Lucio Ponciano Gomes, Bob Tribe, and Marina Diteshein for their contributions to this article.







