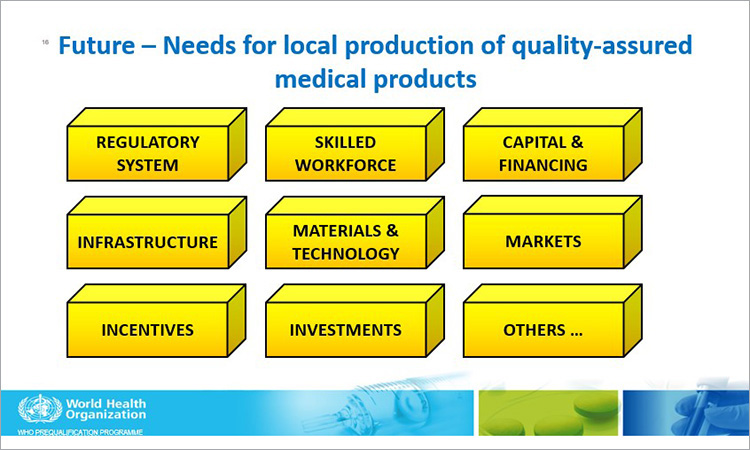
ISPE is committed to fostering communications and interactions to advance common interests among the pharmaceutical industry and regulatory agencies.
ISPE’s Advancing Pharmaceutical Quality (APQ) Program Fully Launched
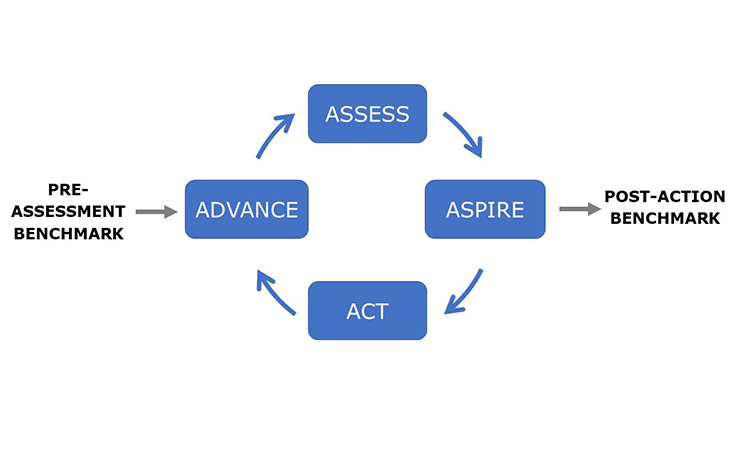
The APQ Program is a quality management maturity assessment built by industry experts for use by industry that provides a practical set of tools and systematic approaches for organizations to advance the effectiveness of their PQS. Read More
Related: ISPE’s APQ Program was presented at an FDA Advisory Council Meeting on Quality Management Maturity on 2 November 2022.
ISPE Highlights Pharma 4.0 at PIC/S 50th Anniversary Event
ISPE’s presentation on its Pharma 4.0™ program was warmly received by the PIC/S participants which included Participating Authorities and Applicant Authorities, Partner Organizations, and invited industry organizations. Read More
ISPE Holds Expert Xchange: Regulatory Summit on Modernizing Inspections
Industry representatives and regulators from six Health Authorities discussed the challenges that must be overcome when using remote technologies, as well as potential mechanisms for enhancing efficiency including international collaboration and reliance. Read More
ISPE Singapore Affiliate Regulatory Panels
A panel of international regulators discussed alternative ways of doing GMP inspections and relying more on each other to make determinations on the GMP status of manufacturers of medicines. Read More
ASEAN Regulatory Roundtable: The Future of Pharmaceutical Inspections

A roundtable of regulators from the WHO, HSA (Singapore) and NPRA (Malaysia) discussed the future of pharmaceutical inspections and recent initiatives that promote regulatory reliance. Read More
Regulatory Insights from 2022 ISPE Annual Meeting
Opening Keynote: Dr. Michael Kopcha, USFDA/CDER, traced the changes in FDA regulation of quality over the years, evolving from reactive approaches to ones that promote mature quality management practices and continuous improvement. Read More
2022 ISPE Annual Meeting: Convergence and Harmonization Support New Therapies
Global Regulatory Town Hall: Following a keynote by Dr. Peter Marks, USFDA/CBER, regulators from seven Health Authorities responded to questions on leading edge modelling capabilities and data review, how approaches to risk management can help or impede adoption of patient-focused risk-based product development and lifecycle management, and whether divergence in control strategy can be avoided. Read More
Regulatory Insights from 2022 ISPE Annual Meeting
Technical Sessions: ISPE’s regulatory committees and working groups presented technical sessions on New Analytical Paradigm (ICH Q2[R2] and Q14); ICH Q12 Implementation; Regulatory Challenges in ATMPs; Quality Management Maturity; Transportable Small Footprint Manufacturing; and Drug Shortages prevention, and more. Read More
ISPE Comments on Draft Guidelines
ISPE provides the opportunity for members to propose comments on selected draft regulatory guidelines or guidances. Commenting opportunities are published in the Regulatory and Quality Networking Community.
ISPE recently submitted comments on: