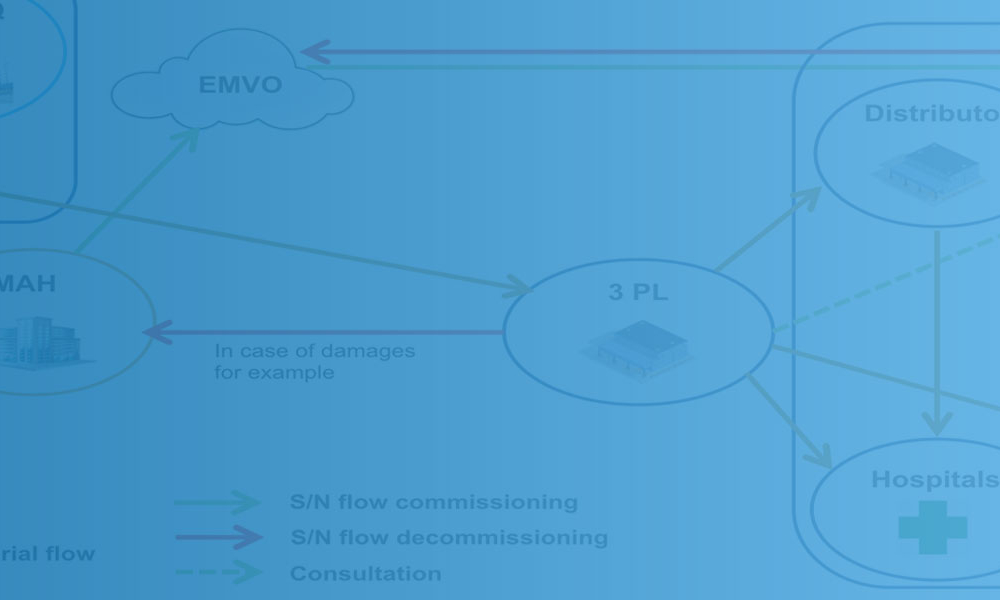
To meet the EU serialization deadline on 9 February 2019, pharmaceutical companies and their contractors have had to reorganize their manufacturing lines and logistics to ensure compliance with the EU’s Falsified Medicines Directive (FMD) of 2011 and the EU Commission Delegated Regulation 2016/161 of 2016. Worldwide, other anticounterfeiting regulations are already in place or coming soon in...

Downloads
A control strategy developed using a science- and risk-based approach will ensure that reproducible product quality is achieved throughout the product life cycle, while maintaining the efficiency, robustness, and flexibility that continuous manufacturing has to offer.
Holistic Control Strategies for Continuous Manufacturing
Cover: A control strategy developed using a science- and risk-based approach will ensure that reproducible product quality is achieved throughout the product life cycle, while maintaining the efficiency, robustness, and flexibility that continuous manufacturing has to offer.
Process Validation in the Context of Small-Molecule Drug Substance and Drug Product Continuous Manufacturing Processes
Features: Highlights from a recently published Discussion Paper detail unique aspects of continuous manufacturing related to each stage of the process verification life cycle.
Regulatory Progress in Global Advancement of Continuous Manufacturing for Pharmaceuticals
Features: Regulatory agencies are reviewing each continuous manufacturing application based on its individual merit, using a science- and risk-based approach to assess the manufacturing process and the product characteristics. This nonprescriptive approach drives innovative, creative thinking and supports the continued growth of CM for small- and large-molecule applications.
Ten Frequently Asked Questions About Serialization
Features: Drug manufacturers that market products internationally must continue to reorganize their operations to comply with many different standards