At the upcoming in-person and virtual 2022 ISPE Aseptic Conference, 14-15 March 2022, you can learn first-hand how people within our industry have faced these challenges and what solutions they have employed to successfully manage them. The program is filled with in-depth presentations and interactive panel discussions with industry and regulatory experts, offering attendees multiple opportunities to gain insights to take back with them. The educational sessions and the networking opportunities with professionals from around the world give you a unique chance to discuss more deeply the current activities and problems you may be facing in your daily work life. Not to forget the adjacent exhibition where you can directly contact companies involved in aseptic processing.

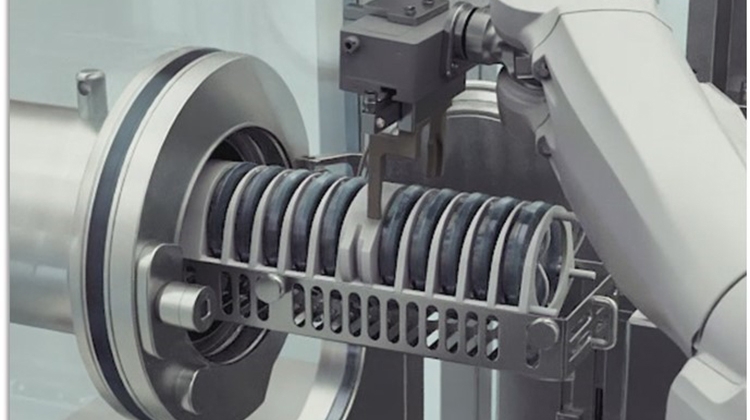
For quite some time, my special interest has been the use of robotics, especially in the area of/fill finish. I am glad to be the session leader of two robotics tracks. Some years ago, we had a dedicated track at the 2017 ISPE Aseptic Conference that included interactive panel discussions with machine suppliers and engineering companies. The interest was huge; I could sense that. In 2018, I moderated a workshop about robotics, and the engagement of the attendees was amazing. We generated many ideas. To name a few: troubleshooting by a robot, fully automatic size part change, and settle plate handling by a robot. In the meantime, I am convinced that for most of the tasks the following statement applies, “What can be automated, will be automated,” because there is a need to keep the impact of human beings out of the process–either to protect the product or to protect the operator, or a combination of both.
At the 2021 Aseptic Conference (virtual), Rick Friedman, Deputy Director at the FDA pointed out that the, “Use of robotics in aseptic processing has the potential to profoundly reduce contamination risks”. The technology is there. And it is getting cheaper and easier to apply day by day.
At the 2022 ISPE Aseptic Conference, you will have the opportunity to hear case studies from the well-known pharma companies, and you can directly address questions to the users of the technology. The topic will be tackled from different angles:
- On the level of designing a whole facility showing the fill finish part but also the supporting areas, automated transfers between cleanroom classes, line set up, cleaning of the rooms and fully automated lab.
- Continuous environmental monitoring strategy that avoids human interventions. Including Qualification and Validation requirements.
- Review of production data from a number of companies like airborne particle data, viable monitoring, media fills and particulate in drug product in order to better understand the benefits of a gloveless, robotic isolator and the resulting risk reduction.
- Retrofit of a legacy line in a way that multiple manual tasks can be performed by the robot instead of the operator.
In the end, the presentations and conversations held at the conference have the potential to revolutionize the complete manufacturing process, with the fully automatic plant on the horizon.
Overall, I am very proud of being part of the conference committee (by the way, I’d like to mention that we have received the 2020 ISPE Committee of the year award) and of the program we can offer. Especially under the circumstances where our traveling is limited, and only on rare opportunities can we meet each other in person. My Program Committee colleagues and I have worked very hard to bring to you a comprehensive technical program to address your company’s challenges and aspirations for the development of your aseptic processes. We are all looking forward to seeing you—hopefully in person!
Explore Agenda & Register
By Klaus Ullherr, Image: Syntegon Technology GmbH