iSpeak Blog
Read, Learn, Innovate: Pharmaceutical Engineering® Revisit Past Article of the Year Winners
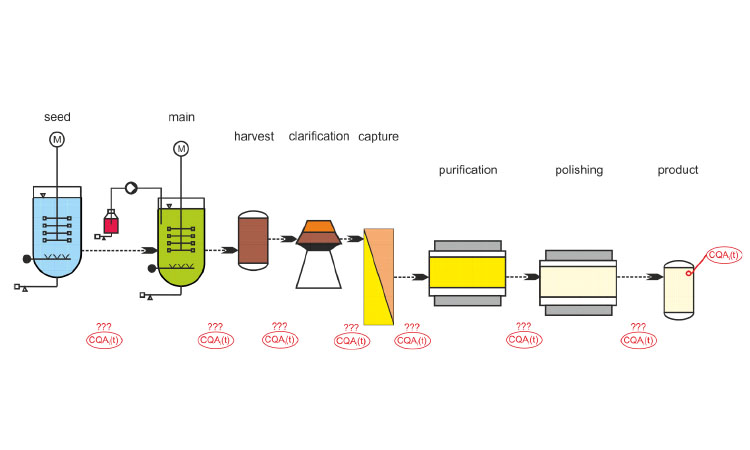
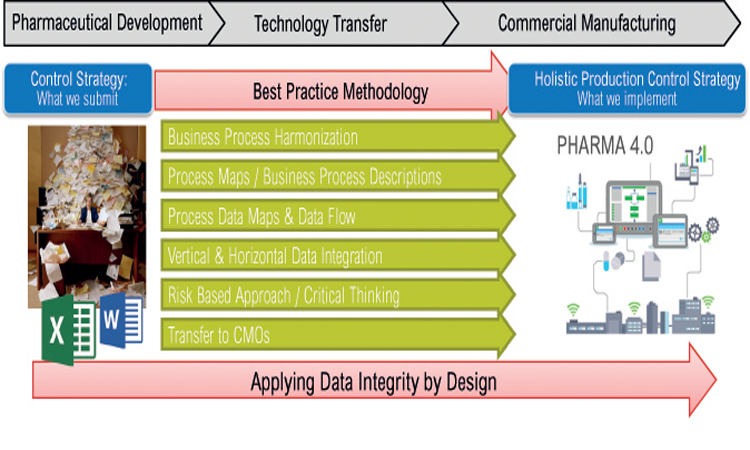
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are a look back at past winners of the Roger F. Sherwood Pharmaceutical Engineering Article of the Year Award.