Exploring the Risk-Knowledge Infinity Cycle (RKI Cycle) across the Product Lifecycle
Case studies demonstrating the link between knowledge and risk in technology transfer and commercial manufacture
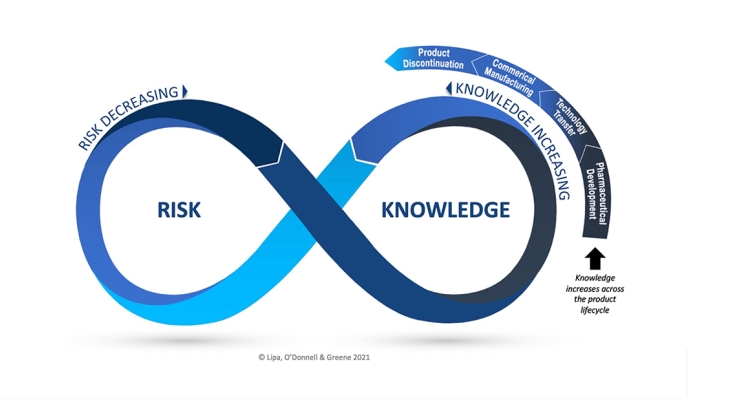
Case studies demonstrating the link between knowledge and risk in technology transfer and commercial manufacture
Since the publishing of ICH Q10, Quality Risk Management (QRM) and Knowledge Management (KM) have been positioned as dual enablers to an effective Pharmaceutical Quality System. While there is broad agreement QRM and KM are highly interdependent in principle, there is recognition they are not well integrated in practice, and better integration is an opportunity to better manage risk and thus, increase patient protection.
A framework, the Risk-Knowledge Infinity Cycle (RKI Cycle), has been developed as a result of a research study carried out in conjunction with TU Dublin, to better integrate QRM and Knowledge Management which can be applied across the entire product lifecycle. Details of the framework will be explored in an upcoming ISPE webinar, and application of the Risk-Knowledge Infinity Cycle Cycle to the Commercial Manufacturing and Technology Transfer lifecycle phases will be illustrated through multiple short case studies, including examples to demonstrate the benefits of effective tacit knowledge transfer. In addition, a re-imagined PQS foundation will presented, enabled by QRM and Knowledge Management as intentionally connected and synergistic practices, as a means to reduce risk and ultimately benefit patients.
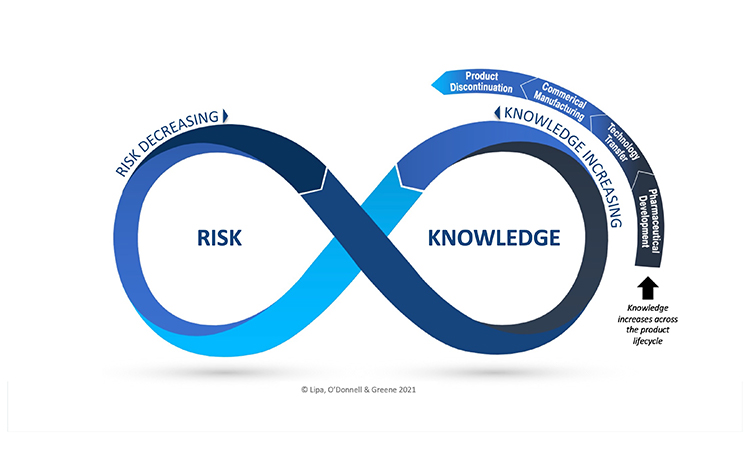
Check out the complimentary webinar Exploring the Risk-Knowledge Infinity Cycle (RKI Cycle) Across the Product Lifecycle on May 20, 2021 at 1100 ET which will also introduce the new ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry.
The new ISPE GAMP® Guide: Artificial Intelligence provides a comprehensive, state-of-the-art best practice framework to efficiently and effectively achieve high-quality AI-enabled computerized systems in regulated life science areas. It bridges established GAMP concepts with...
Each year, FOYA showcases innovation, excellence, and progress in the pharmaceutical industry. The winners exemplify state-of-the-art design, cutting-edge technology, and industry best practices, continually setting new standards for what constitutes a winning project. This constant evolution keeps FOYA fresh and original, making it all the more remarkable that FOYA is celebrating its 20th...
