AI/ML Technology: Harnessing AI/ML Technology for the CQV Life Cycle

Technology is advancing at an extraordinary rate. Industries are benefiting from automation and AI. As technological developments continue to reform the way industries run, the integration of AI and machine learning technologies in the life sciences industry is redefining the traditional approach to commissioning, qualification, and validation (CQV) in pharmaceutical manufacturing.
As the life sciences industry becomes increasingly more regulated, complex, and dynamic, so does the CQV life cycle. Traditional CQV approaches often fall short, but implementing AI/ML can revolutionize the CQV life cycle.
CQV requires huge efforts with the expectation to turn over equipment, systems, and facilities promptly for manufacturing use. This involves generating deliverables that will be used throughout the life cycle of the equipment or system within a stringent timeframe. The speed at which deliverables are generated carries the potential risk of human error during document generation, execution, and turnover management.
AI/ ML technologies are appearing as a notable change in CQV by streamlining, automating, and optimizing various stages of the life cycle.3 Machine learning applications can significantly accelerate the CQV phase by automating the analysis of validation data, finding trends, and predicting potential issues and anomalies that may go unnoticed by human observers.
ML algorithms can adapt to changing conditions, improving the agility of validation processes. This predictive capability allows for proactive decision-making, reducing the risk of unexpected issues.4 As we navigate through this combination of innovation and established industry practices, the influence of AI/ML offers a better future, where efficiency and compliance combine to optimize the CQV processes.
By harnessing the power of AI/ML, CQV has the potential to overcome challenges in data analysis, automated workflow creation, anomaly detection, risk assessment, and predictive modelling. The technology is superior in optimizing the commissioning process, integrating different systems and components enabling predictive maintenance, and supporting decision-making processes—all in a much faster and compliant manner as compared to humans. It can improve overall efficiency, accuracy, and effectiveness of the CQV process.
The transformation of the CQV life cycle using AI/ ML technology will streamline and accelerate the overall process leading to improved outcomes significantly enhancing productivity, reducing timelines, and boosting overall effectiveness of the project life cycle.
Background
CQV serves as a foundation within the pharmaceutical manufacturing industry to ensure the integrity and reliability of the manufacturing processes. “Commissioning” is the process of ensuring that a system or facility is designed, installed, tested, and is operating according to its intended specifications which accounts for Good Engineering Practices (GEP). “Qualification” is the process of verifying and documenting that equipment, systems, and processes are installed and operate correctly and consistently within established specifications. “Validation” is a process of establishing documented evidence that a system or process consistently produces results meeting predetermined specifications and quality attributes. Qualification and validation account for Good Manufacturing Practices (GMP).
This article explores how AI/ML technologies can transform the CQV life cycle. As technological developments continue to reform the way industries operate, the integration of AI/ML technologies pave the way to redefine the traditional approach to CQV.
AI/ML technologies account for some noteworthy changes in CQV by streamlining, automating, and optimizing stages throughout the CQV life cycle.3 ML applications can significantly accelerate the CQV phase by automating the analysis of validation data, finding trends, and predicting potential issues, and anomalies that may go unnoticed by human observers. ML algorithms can adapt to changing conditions, improving the agility of validation processes. This predictive capability allows for proactive decision-making, reducing the risk of unexpected issues.4 As we navigate through this combination of innovation and industry established practices, the influence of AI/ML offers a better future where efficiency and compliance seamlessly coexist to redefine the excellence in the CQV process.
Revolutionizing the CQV Life Cycle
The pharmaceutical industry is experiencing a paradigm shift with the integration of AI/ML into the CQV life cycle. CQV is a critical step in ensuring that manufacturing processes and systems are properly designed, implemented, and maintained to produce safe and effective products. Conventional CQV methods often involve manual testing and extensive documentation, leading to extended timelines, inefficiencies, and potential errors. Because AI/ML technologies can evaluate vast amounts of data quickly and find patterns or variances, they help in identifying and addressing gaps that might be missed by operators and reviewers.
Another crucial benefit of implementing AI/ML in CQV is enhanced data analysis capabilities by enabling real-time monitoring of process and equipment. This is done by collecting data from various sources such as sensors or electronic records. This allows for proactive identification of issues before they turn into a costly downtime.3 By harnessing the power of AI/ML technology, we can address challenges, making the workflow smarter, more adaptive, and efficient. Therefore, the usage of AI/ML technology in the CQV life cycle offers significant benefits and enhances overall efficiency and effectiveness.
Translating Need to Blueprint
Recognizing the need for AI/ML technology enables the design and implementation of solutions. As a prerequisite, a complete understanding of the process, equipment, supply chain, and data quality compliance are essential to having a strong design basis.5 A multidisciplinary approach with domain expertise is required for collective functioning, tailoring unique solutions and mitigating challenges.6
Because data and records in the pharmaceutical industry are highly critical, sensitive, and subjected to regulatory reviews, selection of advanced sensors, instruments and field devices that can integrate and exchange data at all levels (i.e., from field level to supervisory or management level) are required. This is because data plays a crucial role for real-time monitoring and control at different stages of the process.3 Other considerations, such as robust data storage systems and infrastructure, are crucial for managing large data volumes from equipment and systems. Additionally, a clear data management strategy is essential to maintain data reliability and integrity.3, 6
When integrating or implementing AI/ML technology with existing infrastructure, several factors should be evaluated for further prospects.4 These include understanding the current level of automation, layers of data exchange, integration with different systems (e.g., laboratory information management systems and inventory management systems) and the capability of other information technology/operational technology (IT/OT) infrastructure to adapt this change. If the current infrastructure can support a change, then the data from various levels, equipment, and IT/OT infrastructure can be collated according to the requirement to create a data source for an AI/ML model.
If the existing field devices’ infrastructure does not support the AI/ML implementation, the level of change required can be assessed with a detailed implementation strategy. The level of change could range from upgrading the existing instruments at the field level to operational requirement changes at the supervisory/management level. This depends on the current condition and the expected state proposed to achieve using AI/ML technology. 2
Because AI/ML technologies can evaluate vast amounts of data quickly and find patterns or variances, they help in identifying and addressing gaps that might be missed by operators and reviewers.
Intelligent sensors and Internet of Things devices when integrated with AI/ML algorithms, can check system performance in real time, facilitating early detection of deviations from the established limits.3, 5 This enhances traceability and ensures that every step is documented and validated in real time, thereby improving the equipment occupancy. With automation and controls in place, we can achieve consistent results while the operators can be assigned to focus on improving more complex aspects of validation. In addition to improving efficiency and accuracy, incorporating AI/ML into the CQV life cycle promotes compliance with regulatory standards. Automated systems can ensure that all necessary documentation is complete while adhering to industry guidelines throughout every stage of validation.
Since “data” are key influencers in revolutionizing the AI/ML technology in CQV life cycle, adopting quality by design principles in data collection processes right from the process developmental stage is essential to the success of implementation.7 Hence, focus should be on designing a scalable system with horizontal and vertical integration of the processes to handle large amounts of data, which can be utilized for data analysis, predictions, and more3, 4, 8 throughout the life cycle. This further enhances the quality, safety, and efficiency of the process. A clear, strategic approach that combines factors is essential for successful implementation of AI/ML in this highly regulated industry..6, 7 These factors include deep understanding of process/equipment, infrastructure/technical possibilities, technology advancement, cross-functional domain expertise, and regulatory principles.
A Hierarchical Approach to CQV Life Cycle Using AI/ML
The hierarchical approach to CQV life cycle using AI/ML, as shown in Figure 1, employs the automation pyramid, which is followed by the manufacturing plant.5 The data and/or signals collected from the equipment or system in field via sensors and actuators are processed into actionable insights as per the requirements specified at each level.6 Huge amounts of data are continuously generated by sensors, controllers, and recorders in the equipment or system.
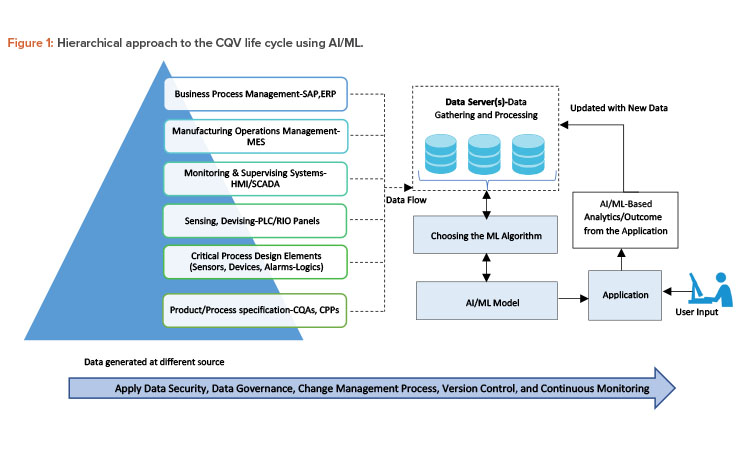
Data from these flows into the data server and/or historian, which serves as an input for AI/ML algorithm and model development. The AI/ML model processes this data, which contains massive amounts of information. This helps in analytics, performance evaluation, modeling, and decision-making. It also provides new opportunities to find patterns and solve difficult conditions that have never been seen or correlated before.8 This enhances the process and improvement actions during the CQV phase and routine operations.
To improve the overall process or system, it is important to focus on the individual steps or unit operations. Changes made at the individual level will affect the performance of the overall manufacturing process and/or system. To have effective control and efficient process, it is important to have correct, reliable, and quality data at each step.5 This helps in thorough analysis and develops an understanding of correlation of how changes made to unit operation or system parameters are contributing to the improvement of overall system and manufacturing processes. Hence, the key to effectively manage and improve a system lies in having quality data at each step of the process that serves as an input to AI/ML models.
Over time, the AI/ML model will be acquired with more data, and it will continue to improve its understanding based on the data feedback loop. The ability of the AI/ML model to iteratively learn over time depends on the type of approach and algorithm used. This ability to learn from vast data and experience and improvise the process over time is the key characteristic of the AI/ML system. It also enables the model to handle complex tasks and provide predictive suggestions at a much faster pace than human beings. Such applications of AI, ML, robotics, and other advanced technologies to perform CQV activities that are traditionally carried out by human operators is called autonomous CQV.6
Autonomous CQV refers to a modern approach within the pharmaceutical industry that involves technologies like AI/ML, robotics, and automation streamlining and enhancing the CQV processes. This includes document generation, execution, data collection, analysis, and reporting carried out with minimal human intervention. The aim is to minimize human error, increase productivity, and ensure compliance with regulatory standards throughout the life cycle of the equipment or system.
Application and Benefits of AI/ML
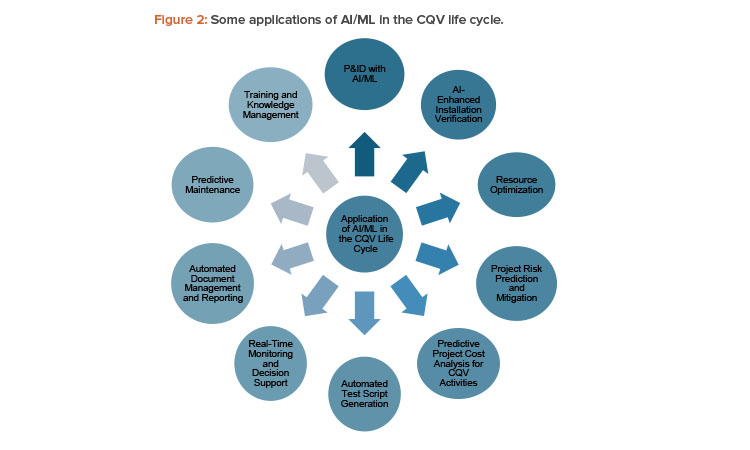
Implementation of autonomous CQV by integrating AI/ML can have a significant impact in improving process knowledge, efficiency, accuracy, and compliance. Some applications are depicted in Figure 2.
Piping and Instrumentation Drawing (P&ID) with AI/ML
Requalifying existing brownfield projects can pose challenges, particularly when P&ID walkdowns are hindered by a lack of accurate “as-built” P&IDs. The traditional method of creating or updating P&IDs relies on manual labor when draftspersons physically navigate the site to convert observations into computer-aided design (CAD) drawings. However, this manual approach often leads to inaccuracies, delays in the requalification processes, and high associated costs. Introduction of the AI/ML-based robot revolutionizes P&ID development with its advanced technology.
During site walkdowns, these robots employ cameras or scanners to collect reliable 3D data, ensuring precise documentation that mirrors actual site conditions. An AI/ML-based robot can be deployed even in confined spaces. It offers a comprehensive 360-degree view through remote access, driving significant time and cost savings by streamlining data collection and reducing manual efforts, thus minimizing rework during project execution.
The in-built 3D scanners in the robot produce detailed point cloud data that can be imported into various CAD software. Moreover, this technology enhances design visualization, aids decision-making, facilitates conflict resolution, and ensures regulatory compliance, ultimately saving time, resources, and promoting sustainability by minimizing waste.
AI-Enhanced Installation Verification
AI revolutionizes equipment installation verification during CQV processes. Through automated image analysis, AI algorithms meticulously scrutinize the actual site condition/installation images or videos by comparing them against predefined trained data sources like installation manuals or videos and CAD models. This help detect errors such as misalignment or missing components. Real-time monitoring powered by AI ensures prompt anomaly detection by analyzing sensor data during installation, enabling swift corrective actions.
AI-driven computer vision systems visually inspect installations, capturing errors like loose connections or damaged components, with remarkable precision. Predictive analytics models forecast potential issues based on historical data, facilitating proactive measures to prevent errors or malfunctions. AI streamlines the compliance process by automating verification, reducing human error, and enhancing accuracy, efficiency, and safety in equipment installation processes, ensuring strict adherence to standards.
Resource Optimization
ML algorithms can analyze project requirements, resource availability, and historical data to optimize resource allocation throughout the CQV process. By accurately matching personnel, equipment, and materials to specific tasks and projects, organizations can minimize idle time, improve resource utilization, and reduce costs, thereby enhancing schedule efficiency and cost-effectiveness.3, 5
Project Risk Prediction and Mitigation
AI/ML techniques can analyze project data to anticipate risks and forecast their probability and consequences on schedule and expenses. These algorithms evaluate project risks by scrutinizing factors like project complexity, resource availability, and regulatory demands. Through proactive identification and resolution of risks, organizations can implement mitigation strategies, leading to smoother execution, reduced rework, and reducing the possibility of schedule extensions.9, 10
Predictive Project Cost Analysis for CQV Activities
AI/ML-based predictive modeling techniques forecast CQV project costs by considering factors like project size, complexity, regulations, and location. These models learn from past data, update predictions with new information, and continuously improve accuracy over time. They analyze feedback and performance metrics to refine predictions and adapt to changing requirements and market conditions. AI can conduct scenario analysis to evaluate the impact of different project variables on overall costs. By simulating various scenarios and adjusting parameters such as project scope, timeline, or resource allocation, AI can help project stakeholders make informed decisions to optimize cost-effectiveness.
Automated Test Script Generation
AI/ML algorithms can analyze historical data from previous validation projects and provide inputs to generate optimized test scripts automatically. These scripts can be tailored to specific equipment or systems, reducing the time and effort required to develop testing protocols from scratch. By automating this process, organizations can significantly accelerate the validation timeline and minimize associated costs.
ML models further aid in adjusting test cases as the system evolves, ensuring ongoing relevance of validation efforts. Adhering to change management processes is crucial for maintaining system integrity, including assessing and implementing ML model modifications effectively. Monitoring the updated model’s performance ensures it meets expectations, with robust version control and tracking mechanisms ensuring transparency and accountability throughout the equipment life cycle.4, 6
Real-Time Monitoring and Decision Support
AI-driven analytics platforms can analyze large volumes of data in real time from validation activities, providing insights into validation processes, equipment performance, and potential issues, which supports CQV teams in their decision-making. For example, these AI-driven systems can flag deviations from expected performance metrics, recommend corrective actions, or alert personnel to potential compliance issues. By leveraging these insights, decision-makers can identify areas for optimization, anticipate challenges, and make informed decisions to streamline the CQV process.3, 4
Automated Document Management and Reporting
AI-powered document management systems use natural language processing algorithms to automate various tasks related to document organization, retrieval, traceability, and review of CQV documentation. These systems can extract relevant information from test results, equipment logs, and other sources to generate reports automatically. By automating these tasks, manual work is reduced, human errors are minimized, and the documentation process is accelerated. Ultimately, this streamlines the CQV process and improves the overall audit trail.
Predictive Maintenance
AI/ML can be employed in predictive maintenance to predict failures before they occur by analyzing sensor data and historical maintenance records. By implementing predictive maintenance strategies, organizations can minimize unplanned downtime, prevent costly equipment failures, and optimize maintenance calibration schedules to maintain the validated state of the equipment and systems. This leads to significant cost and time savings.
Training and Knowledge Management
AI/ML models can adapt the learning path based on the individual’s progress, performance, and interest for a customized experience. Integrating virtual reality and augmented reality technologies can create realistic and immersive training experiences.9 AI-powered chatbots can provide immediate assistance and respond to queries related to training materials. This leads to organizational growth and more efficient, personalized, and adaptive learning experiences for employees.6
Challenges and Considerations
AI/ML in CQV processes can offer numerous benefits, but it also presents several challenges and considerations..5, 6, 10, 13
Challenges
Data quality and quantity
AI/ML models require large volumes of high-quality data to train effectively. Obtaining sufficient historical data for CQV processes may be challenging.
Data integrity and security
Ensuring the integrity and security of data used for AI/ML models is critical. This is particularly true in regulated industries where data integrity is paramount for compliance with regulations, such as the US FDA’s 21 CFR Part 11.14 Maintaining data integrity throughout the CQV life cycle is essential.
Data privacy
Stringent security measures are crucial when handling sensitive data in CQV processes to prevent inadvertent disclosure. It also safeguards organization, equipment, and personnel information, which prevents privacy breaches of information used in AI/ML models. Compliance with data privacy regulations preserves data utility for AI/ML model training, and robust data sharing agreements with third-party vendors prevent unauthorized access.
Model interpretability and explainability
AI/ML models often operate as black boxes, making it difficult to understand the reasoning behind their decisions. In regulated industries, it’s crucial to ensure that CQV processes are transparent and understandable, which may require using interpretable ML models for developing methods to explain model predictions.
Regulatory compliance
Adhering to regulatory requirements is a fundamental aspect of CQV processes. Introducing AI/ML into these processes requires careful consideration of regulatory guidelines and standards, such as the US Food and Drug Administration (FDA)’s validation requirements for computerized systems and the European Medicines Agency’s guidelines. 11
Validation of AI/ML models
Validating AI/ML models for use in CQV processes requires demonstrating their reliability, accuracy, and consistency. Establishing validation protocols and methodologies specific to AI/ML models can be challenging due to their complexity and dynamic nature.
Considerations
Data selection and management practices
Establish a comprehensive strategy to collect important data, making sure all necessary sources are identified and available to train and validate the AI/ML models effectively. Data sources can be diverse and tailored to meet the specific needs, each presenting unique considerations. These sources range from structured to unstructured data.
Risk assessment
Conducting a comprehensive risk assessment is essential to identify potential risks associated with the use of AI/ML in CQV processes. Understanding these risks allows organizations to implement appropriate risk mitigation strategies and controls.
Expertise and training
Building internal expertise in AI/ML and providing training for personnel involved in CQV processes are critical considerations. Organizations may need to invest in training programs to ensure staff have the necessary skills to develop, implement, and maintain AI/ML-based systems.
Collaboration and stakeholder engagement
Collaboration between cross-functional teams, including quality, manufacturing, IT, engineering, and regulatory affairs, is essential for successful integration of AI/ML into CQV processes. Engaging stakeholders throughout the process helps ensure alignment with organizational goals and regulatory requirements.
Continuous improvement and monitoring
Implementing AI/ML in CQV processes requires a commitment to continuous improvement and monitoring. Organizations should establish mechanisms to monitor model performance, address issues as they arise, and incorporate feedback to refine and optimize AI/ML systems over time.
Ethical and social considerations
Ethical considerations, such as bias in AI algorithms and the potential impact on workforce dynamics, should be carefully evaluated. Organizations must ensure that AI/ML systems are developed, deployed, and maintained in a responsible manner, aligning with ethical principles and societal values, as defined in ISO 42001 standard.12
AI-powered document management systems use natural language processing algorithms to automate various tasks related to document organization, retrieval, traceability, and review of CQV documentation.
Data governance
Privacy by design is crucial in AI/ML system development. Principles like data minimization and purpose limitation reduce privacy risks by ensuring that only necessary data is collected. Transparent communication about data practices fosters trust and accountability. Obtaining informed consent from users is essential. Robust data encryption and access controls enhance data security. By adopting privacy-enhancing technologies, organizations can mitigate privacy risks and build trust in their AI/ML initiatives. By tackling these challenges, organizations can use AI/ML to improve efficiency, accuracy, and compliance in CQV processes while reducing risks and ensuring regulatory adherence.
Conclusion
AI/ML revolutionizes the CQV life cycle, boosting efficiency, accuracy, and innovation to meet evolving demands effectively. Traditional approaches fall short, leading to inefficiencies and higher costs. Applications like AI-/ML-enabled resource optimization, P&ID development, installation verification, and automated test script generation drive productivity, quality, and cost-effectiveness improvements by optimizing resource allocation. Proactive risk prediction and mitigation prevent delays or failures, and predictive project cost analysis aids resource allocation. Real-time monitoring offers actionable insights for compliance and performance optimization.
Automated document management and reporting ensures regulatory compliance, and predictive maintenance maximizes equipment uptime. AI/ML-driven training fosters continuous learning, empowering organizations to thrive in a dynamic environment. Yet challenges such as data integrity, security, organizational readiness, and cultural adaptation persist. Collaboration among industry stakeholders, regulatory bodies, and technology providers are vital to surmounting these obstacles and unlocking AI/ML’s full potential in CQV. Organizations must stay vigilant, adaptable, and dedicated to driving positive change as they embark on this transformative journey. With AI/ML, we can revolutionize the CQV life cycle, attaining unparalleled efficiency, agility, and quality while shaping future industry standards and innovation.



