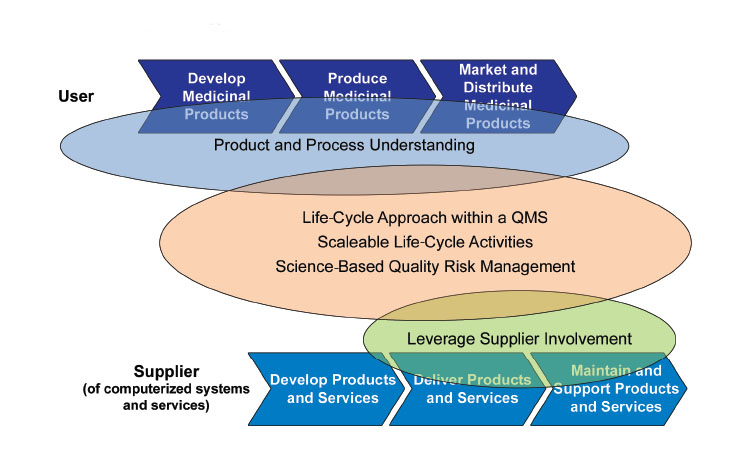
The pharmaceutical industry is experiencing a pivotal transformation. As professionals strive to develop therapies faster, more cost-effectively, and with greater precision, Pharma 4.0™ emerges as a powerful framework for change. This evolution is not only technological—it is cultural. It challenges organizations to become more agile, data-driven, and patient-focused.