Digital Labels Revolutionize Investigational Medicinal Products (IMPs)

Labeling is an important part of the supply chain. This is especially true for investigational medicinal products (IMPs), which must be labeled with clear expiry dates and other mandated information. IMP shelf life is notoriously difficult to quantify, however, and new findings on their stability frequently emerge during clinical trials. This often requires companies to relabel IMPs with revised expiry dates and other information. It's an expensive and time-consuming process that affects the entire supply chain.
Whenever stability data change, we typically initiate a relabeling process where IMPs receive new labels that carry revised expiry dates,” Rocco Barone, Associate Director, Operations, at Merck & Co., Inc., told Pharmaceutical Engineering. “Relabeling takes time and can lead to supply chain delays and budget extensions. IMPs may need to be shipped from the trial site to the depot, where they are relabeled, before being returned to the site. The complete relabeling process not only means time and cost. Compliance requirements for manual relabeling are also high,” he said.
According to a study conducted by the Tufts Center for the Study of Drug Development, the US Food and Drug Administration (FDA) approved 225 new biopharmaceutical products from 2006 through 2016, accounting for 35% of all new drug approvals during that period. And the pace is increasing: The FDA approved 13 new biotech products annually during the first eight years of the study, but that figure climbed to more than 20 per year between 2014 and 2016, with a market value of $68 billion in the final year.
It’s no surprise that the share of biopharmaceutical IMPs in clinical trials is rising steadily. Over the next two decades, 70% of traditional medications will be replaced with biopharmaceuticals, making IMP labeling methods critical to their predicted growth. “Biopharmaceuticals are complicated and expensive to manufacture,” Barone noted. “In addition, their stability data are often so limited that it is sometimes impossible to conduct any trial at all.”
Paper Labels, Manual Updates
While smart labels with radio frequency identification (RFID) tags have become a fixture in pharmaceutical manufacturing, the IMP labels used in clinical trials have not been able to adopt the technology as readily. As clinical trials become more complex and costly, however, finding ways to be make IMP materials more efficient and cost effective is imperative.
Traditional paper IMP labels, for example, must include multiple regulatory elements. To meet these requirements and fit the information into the small space available (on a syringe or vial, for example), providers are often forced to resort to very small, nearly illegible font sizes. And when expiry data change—an especially frequent occurrence with biopharmaceuticals—each package must be manually relabeled, a costly and time-consuming process.
“The current method for updating reevaluation dates on clinical labels involves a manual process that leads to inefficient use of resources and capacity, with long cycle times,” Barone told attendees at the ISPE 2017 Annual Meeting & Expo in San Diego, California. “Total time is approximately three to five weeks.”
Digital technology, however, is helping clinical trials find new ways to incorporate faster and lower-cost labeling options for IMPs. Electronic labels (e-labels) can contain a regulatory-compliant IMP label, and they may also decrease or even eliminate deviations caused by extension relabeling, sterility breaches, tamper-evident seal removal, product mix-up, and temperature excursions. In addition, IMP changes that affect label content can be communicated quickly.
Digital technology is helping clinical trials find new ways to incorporate faster and lower-cost labeling options for IMPs.
The Evolution of Smart Labels
Unlikely as it may seem, the first e-label was patented in 1952. While functional, it remained too expensive for large-scale implementation for over 20 years. IBM adapted the technology in 1973, and the result was the ubiquitous one-dimensional bar code we now know as the Universal Product Code, or UPC (Figure 1). By 1980, the symbols and their scanning systems were being adopted by 8,000 stores per year.
Since their debut in grocery stores, bar codes have found a broad range of uses in the pharmaceutical industry, including drug manufacturing, security, identification, traceability, and expiry information.
Two-dimensional (2D) bar codes were introduced in 1990. These images, which hold significantly more information than their one-dimensional predecessors, can be read vertically and horizontally (Figure 2). They are used on primary and secondary pharmaceutical packaging as part of global serialization initiatives and other health authority guidances. Since January 2011, for example, 2D data matrix codes have been part of European pharmaceutical regulatory mandates.
By the mid-2010s, “smart labels” included RFID technology. RFID tags can hold significantly more information than standard bar codes and can be read inside or outside of the package. Within a year, near-field communication (NFC) labels carried even more information than a 2D bar code and could be read on a smartphone or tablet.

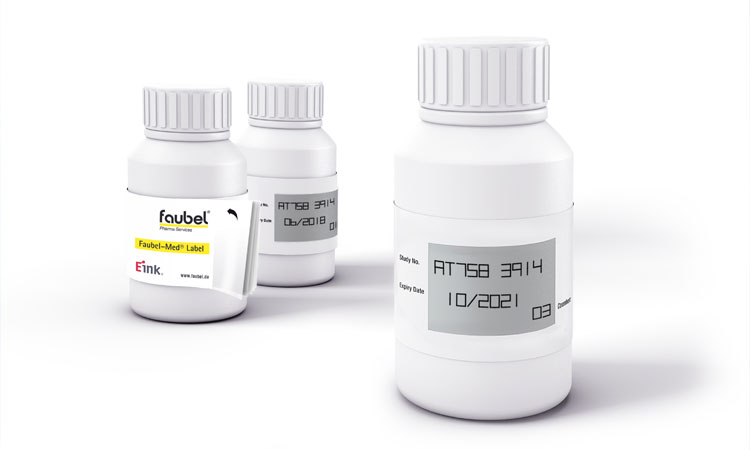
E-Paper Label System
Merck & Co., Inc., in partnership with Faubel & Co. Nachf. GmbH, have co-developed Med Label, a semi-electronic label system that replaces the manual IMP label updating process with a time-saving, cost-effective alternative (Figure 3).
Software reads an RFID tag attached to a standard IMP booklet label. Each RFID tag contains a transponder (a microchip with an antenna or coil), an analog circuit for receiving and transmitting (transceiver), a digital circuit, and a permanent memory. Since RFID tags cannot display information, smart labels are equipped with battery-free e-paper displays linked to the tags. If the transponder is within range of a reader, connection is triggered through electromagnetic induction. Each RFID tag also carries a unique identification number (UID).
RFID UID and GPS location data can be combined to monitor labeled packages across the entire value chain (track and trace). “This kind of monitoring is the key to seamless traceability in clinical trials,” Barone noted. “It also helps control material and product flows in inventory management.”
RFID tags can hold significantly more information than standard bar codes and can be read inside or outside of the package.
* E-paper devices have easy-to-read displays that reflect light to simulate the appearance of natural ink on paper. They consume just 1% of the power required for an LCD display and can be read in direct sunlight.
US Pilot
To evaluate the technology and eliminate regulatory conflicts, Merck conducted US-only trials in September 2016. (The United States does not require reevaluation dates on labels.) Demonstration videos and user manuals helped the studies run smoothly.
“There are a few pharma companies working on these smart technologies in clinical trials,” Meinrad Kopp, Head External Networks Management, Merck, told Clinical Trials Arena in June 2016. “However, I [have] yet to see a company that has run a larger trial with such smart technology for their patients.”
In the trial, each container received a fully compliant paper label plus a Med Label. Merck then ran two extension updates: one within Merck and one at a clinical trial site.
“Compared to conventional relabeling, which often takes months, the label can cut updating time to just one day if everything is well thought through,” Barone explained to Pharmaceutical Engineering. “By eliminating a whole range of work steps, such as filling out documentation by hand, daily work went faster without jeopardizing the quality of work in any way.”
Participants completed and returned questionnaires at the end of studies. Results were positive and contained few suggestions for improvement. Following these successful tests, Faubel launched Med Label in 2017.
In an April 2018 press release, Konrad Zachman, Head of Production for New Technologies at Faubel, said that the label “… is a breakthrough product that makes the handling of investigational medicinal products in clinical trial supply chains so much easier.”
Advantages
Temperature
For many cold-chain IMPs, temperature fluctuations of 2°–8°C can cause quality problems, as can high temperature and humidity levels in the supply chain. Smart labels with integrated RFID temperature sensors can withstand a wide range of temperatures. The update device is rated to –5°C, and the e-paper display can function at –20°C. Depending on the requirements, booklet labels can be made of special paper, foil, adhesives, and inks for temperature and moisture resistance.
Regulatory compliance
Neither the EU guidelines nor the US Code of Federal Regulations Title 21 state that labels must be made exclusively of analog materials such as paper. No requirements prohibit RFID technology in labels, nor are individual components, such as antennas or sensors, covered by these guidelines.
“In the past, authorities had no objection to innovative labeling as long as requirements on contents, durability, and legibility were met,” Barone explained. “A smart label with an RFID tag, e-paper display, and booklet label complies fully with Annex XIII,” he said. Implementing Annex VI may pose a challenge to the pharmaceutical industry.
Tracking and documentation
Because the software stores the UID of each RFID tag, multiple expiry dates and counters inside the kit can be updated in a single operation. As soon as the tag starts to exchange data with the update device, data on individual packages is received in real time.
Data for each labeled container is automatically stored in the software before being merged into batch documentation. This can serve as a source of information for inventory management whenever logistical product flows are to be controlled and monitored.
If an allocation error occurs when the container is shipped from the depot, for example, it can be identified if data transfer is performed using an update device. Wrongly addressed containers can then be withdrawn from the shipment.
Automatic updates
Key takeaways, Barone noted, were the elimination of labor- and resource-in•tensive activities—such as writing batch records, printing and shipping labels, and physically applying labels in depots/sites—as well as vastly improved cycle times.
“Another potential benefit of e-labels,” Kopp told Clinical Trials Arena in his 2016 interview, “is you can start a trial with a very short initial shelf life. Whenever new stability data becomes available, new retest dates can be assigned and an update can be performed, because it is so simple and so quick. It is a matter of minutes.”
Smart labels are equipped with battery-free e-paper displays linked to the tags.
Next Steps
Merck is currently focusing on Phase 1 and 2 studies, Barone said, but hopes to focus on larger Phase 3 studies in the future, and will also explore additional options such as new label formats, study pooling, and full electronic display of a clinical label.
“I can imagine over the next few years we will start to see real trials being conducted with the help of these technologies,” Kopp concluded. “What’s more, I am personally convinced this is the future. Over time, the use of paper labels will gradually fade and we’ll start to see more electronic technology being used in clinical trials.”
References
- Clinical Trials Arena. “The eLabel vs. the Booklet Label.” Supply Chain, no. 27 (7 June 2016). http:// www.clinicaltrialsarena.com/news/supply-chain/the-elabel-vs-the-booklet-label-4915804
- Cognex Manatee Works. “The Evolution of Barcodes: Beyond Barcodes.” 16 September 2016. https://manateeworks.com/blog/2016/09/evolution-of-barcodes-beyond-barcodes
- European Commission, EudraLex, The Rules Governing Medicinal Products in the European Union, EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, Annex XIII [3], Volume 4, 2010. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32014R0536
- European Commission, EudraLex. The Rules Governing Medicinal Products in the European Union, EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use. Annex XIII [3], Volume 4, 2010. https://ec.europa.eu/health/documents/eudralex/vol-4_en
- European Commission, EudraLex. The Rules Governing Medicinal Products in the European Union, EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use. Volume 4, 2010. https://ec.europa.eu/health/documents/eudralex/vol-4_en
- European Commission, EUR-Lex, Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32014R0536
- European Commission, EUR-Lex, Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32014R0536
- European Commission, Guideline on the Readability of the Labelling and Package Leaflet of Medicinal Products for Human Use, Revision 1, 12 January 2009. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-2/c/2009_01_12_readability_guideline_final_en.pdf
- Finkenzeller, Klaus. RFID-Handbook: Fundamentals and Applications in Contactless Smart Cards, Radio Frequency Identification and Near-field Communication, 3rd ed. UK: John Wiley and Sons, 2010.
- Forcinio, Hallie. “Smart Labels Enhance Drug Packaging.” PharmaTech.com, 17 February 2016. http://www.pharmtech.com/smart-labels-enhance-drug-packaging
- International Society for Pharmaceutical Engineering. Good Practice Guide: Booklet Labels. 2013. https://www.ispe.org/publications/guidance-documents/good-practice-guide-booklet-labels
- Nachtrieb, John. “The Present and Future of Barcodes.” Barcode-Test. 20 April 2015. http://barcode-test.com/the-present-and-future-of-barcodes/ (2015)
- Nessim, Doris. “Pharmaceutical Preparation & Product Identification.”GS1 Canada. 24 September 2015. https://www.gs1.org/sites/default/files/docs/healthcare/gs1_canada_hpacwebinar_sept_24final.pdf
- 99% Invisible. “A Short History of the Modern Bar Code.” The Eye: Slate’s Design Blog. 3 April 2014. http://www.slate.com/blogs/the_eye/2014/04/03/a_short_history_of_the_modern_barcode_99_percent_invisible_by_roman_mars.htm
- Pharmafile.com. “High Demands: Requirements on the Labelling of Pharmaceutical Products Are Growing Continuously.” Merck press release, 9 July 2017. http://www.pharmafile.com/content/high-demands-requirements-labelling-pharmaceutical-products-are-growing-continuously
- PharmExec.com. “Tufts CSDD Reports Significant Growth for New Biotech Products.” 9 May 2017. http://www.pharmexec.com/tufts-csdd-reports-significant-growth-new-biotech-products
- SICK USA Blog. “Barcodes: Ringing Up Sales Past, Present, and Future.” 13 July 2015. http://sickusablog.com/barcode-history/
- Smith-Gick, Jodi, Nicola Barnes, and Rocco Barone. “The Near-Term Viability and Benefits of eLabels for Patients, Clinical Sites, and Sponsors.” Sage Journals, 2 April 2018. http://journals.sagepub.com/eprint/6Y3TQ6sz38kNTnmK23Wp/full
- TransCelerate Biopharma, Inc. “eLabels.” http://www.transceleratebiopharmainc.com/initiatives/elabels/
- US Food and Drug Administration, Code of Federal Regulations, Title 21, Part 210-211, 1978.
- US Food and Drug Administration, Code of Federal Regulations Title 21, Part 210-211, 1978. https://www.ecfr.gov/cgi-bin/text-idx?SID=c8513490d92e81f19c036b0fabaa51f2&mc=true&tpl=/ecfrbrowse/Title21/21cfrv4_02.tpl#0