Identifying Global Themes in Clinical Study Participants’
This article examines patient preferences in one facet of clinical research: the experience related to the use of investigational medicinal products (IMPs). As patients have become more involved and informed in their healthcare choices, the “voice of the patient” has been increasingly incorporated into the drug development process. Given that the success of a clinical study relies on the recruitment, retention, and compliance of participants, all stakeholders—sponsors, investigator sites, and clinical study supply providers—need to understand patients’ mindsets and participation experiences throughout a study. Patient-centric knowledge can help improve the investigational process, support adherence, and ultimately create a comprehensive ecosystem for engaging patients who participate in clinical research.
Using data collected from North America, Europe, China, and Japan, this article provides a consolidated analysis, highlighting regional differences and similarities to help clinical trial stakeholders make more informed decisions in the design and implementation of IMPs in global studies.
The Study’s Growing History
Seminal research on patient perceptions of IMPs was first conducted in 2012 by the Patient Survey Project Team at the ISPE’s Investigational Products Community of Practice (IP CoP). With survey results from 1,425 clinical trial participants (predominately in North America), the team analyzed respondents’ opinions about their experiences with IMPs and published suggestions for improvement.1
Although these findings were intended to help improve the patient experience and better align medicine kit design with the needs of the patients, the study’s collaborators wanted to expand the survey to a globally diverse population. With an adapted survey run in 2015, they targeted a larger geographical scope. The team first expanded to Europe and China, publishing those consolidated results in 2016.2 Around the time of that publication, a team in Japan started gathering data from current and past clinical trial participants. The research teams have now received and analyzed data from 1,473 participants in Japan to create new averages with previously collected data in 2017.
Given that clinical supply practices have not drastically changed since the original survey was completed in 2013 in North America, the study collaborators’ combined data can be compared and used as a benchmark across the four regions.
Objectives
With data collected from Japan and compared to data from North America, China, and Europe, the survey sponsors wanted to identify global themes and regional differences as they related to the use of IMPs. Other goals of the survey and its resulting publications included:
- Increasing the industry’s understanding of the patient experience with IMPs
- Determining if there are any noticeable differences in patient experiences
- Providing stakeholders with valuable data sets to support correct decision-making relating to the use of IMPs
- Fostering collaboration between global regulatory agencies, facilitator organizations, and stakeholders involved in the clinical trial process
| North America (2013) |
China (2014/2015) |
Europe (2014/2015) |
Japan (2017) |
|||||
|---|---|---|---|---|---|---|---|---|
| Participating in a clinical trial | Currently | 31% | Currently | 68% | Currently | 40% | 2016 | 45% |
| < 6 months ago | 23% | < 6 months ago | 16% | < 6 months ago | 11% | 2015 | 23% | |
| < 6 months ago | 46% | < 6 months ago | 16% | < 6 months ago | 49% | Before 2015 | 32% | |
| Female | 60% | Female | 43% | Female | 49% | Female | 28% | |
| Male | 40% | Male | 57% | Male | 51% | Male | 72% | |
| Top three therapeutic areas | Diabetes | 12% | Diabetes | 23% | Neurological | 23% | Heart disease* | 40% |
| Respiratory | 9% | Heart disease | 16% | Cancer | 17% | Diabetes | 18% | |
| Pain | 9% | Cancer | 16% | Heart disease | 14% | Hyperlipidemia | 14% |
*(Hypertension, cardiac angina)
Methodology
In each region, the survey reused many of the same questions from the original North America study. However, some questions were eliminated from the surveys that followed in China, Europe, and Japan to focus on key themes recognized in the original survey. Some other questions were slightly reworded to account for cultural differences in translation. The methodology varied among geographic locations as described next. The teams relied on agencies, site management organizations, and patient advocacy groups that had access to patients mostly through clinical trials, pharmacies, and research nurses. Access to appropriate patient populations was instrumental to the survey’s success, with patient anonymity being strictly controlled. See Table A for details about patient demographics.
North America
For North America (N = 1,425), 48 questions in an electronic survey were given to patients that had taken part in a clinical trial in their lifetime and taken their medication home (to ensure a participant was not from an in-hospital study).
Europe
For Europe (N = 109), the study was conducted electronically and in English only, with 48 questions adapted from the original study in North America. The small sample size in Europe was attributed to several factors. First, the survey was delivered only in English, which may not be the primary language of potential participants. Second, as a generalization, Europeans tend to be a little more reserved and are more reluctant to either openly praise or criticize than in North American culture. Third, participants were excluded if they had not participated in a clinical trial that involved IMPs. In addition, not all patients responded to every question; thus, the figures show varying N for the European study population. The results were reanalyzed for this publication; therefore, they slightly differ from the referenced publications.
China
For China (N = 1,935), the survey contained 44 questions modified from the original study in North America, which were translated into Chinese. Data were collected via mobile or paper versions, depending on patients’ preferences. Surveys were conducted in person at study sites.
Japan
For Japan (N = 1,473), the survey focused on 18 key questions, which were designed based on the questions from the survey in Europe. Working with the University of Tokyo, an online survey was sent to 2,688 adult patients (> 20 years old) who enrolled in clinical trials conducted from 2013 to 2016 in Japan. Those patients were extracted from the survey panel of INTAGE, Inc. The data collection period was March 7–9, 2017. The response rate was 54.8%. The survey designers reduced the number of questions to increase the chances that the participants completed the entire survey. The data were collected in an online format.
Results
The following section discusses the results, outlining the range of criteria used throughout the surveys.
| North America (2013) | China (2014/2015) | Europe (2014/2015) | Japan (2017) | |
|---|---|---|---|---|
| Bottle | 42% | 47% | 29% | 14% |
| Blister | 30% | 37% | 37% | 71% |
| Syringe | 15% | 14% | 20% | 12% |
*Other medications included topical and inhaled forms.
- 1International Society for Pharmaceutical Engineering. “Report on the ISPE Project Concerning Patient Experiences with Clinical Trial Materials.” November 2013.www.ispe.org/ patient-initiative/2013novreport.pdf
- 2Sadler-Williams, Esther, Lynn Wang, Samantha Carmichael, and Paula McSkimming. “Patient Perceptions of IMPs: An International Perspective.”Pharmaceutical Engineering36, no. 3 (May/June 2016):
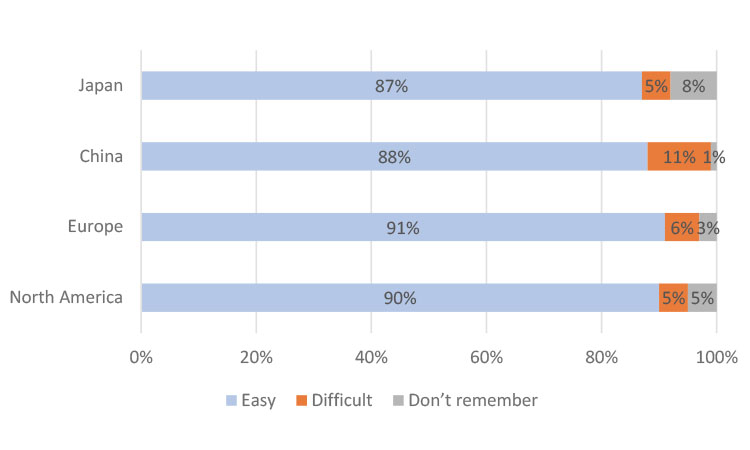
Ease of Medication Use
When asked if it was easy to use their medicine kit, the clear majority of participants in all four studies found their medication “somewhat easy” or “very easy” to use. Each study had a high number of participants that reported their medicine kit was “somewhat easy” or “very easy” to use: 87% for Japan, 88% for China, 91% for Europe, and 90% for North America.
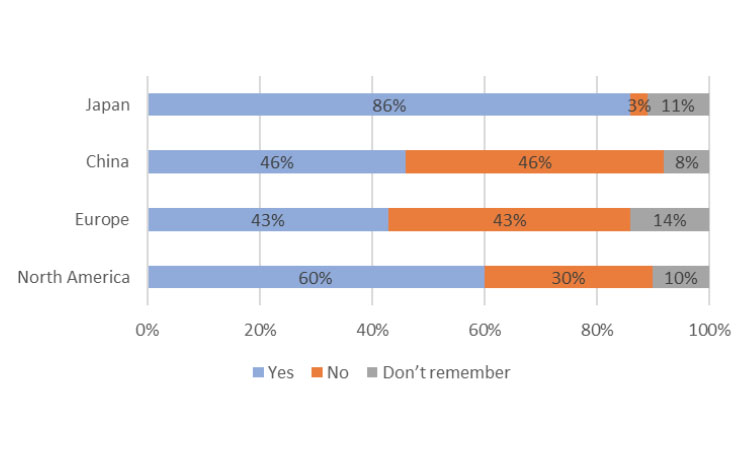
Kit Design
Participants were asked a yes/no question: “Did the design/layout of the medicine kit help you take your clinical trial medicine on schedule?” Although we noted some significant differences in regions (Figure 2), overall, 86% of Japan study respondents stated that the medicine kit design helped take medicine on schedule. For the participants using only bottles (although this was a much smaller percentage of the overall survey total), 91% said that kit design supported taking their medicine on schedule. Thus, in Japan, whether bottle or blister packaging is used, design is a key component to supporting the patient taking their medication on schedule.
The participants in China were split evenly: 46% said the kit design was helpful and 46% said it wasn’t. This could be because the respondents in China heavily value their direct interactions with site staff for medication scheduling, a statement that can be supported by a later question in the survey about reminders to take clinical medication. In that question, 77% said that it was helpful or very helpful to receive “instructions from my physician/nurse/pharmacist every time I visit the hospital or medical center” as reminders for taking a medicine on schedule.
For survey participants in Europe, 43% said kit design was important to taking clinical trial medication on schedule, but the same percentage ( 43%) found kit design unimportant. This was evaluated further by reviewing the top three forms of medication received: blister packs, bottles, and syringes. Of the cohort using blister packs, only 31% answered “yes,” 38% answered “no,” and the remaining 31% answered “couldn’t remember.” Those using bottles had an equal split (45% each) between “yes” and “no”; for those using syringes, 23% said “yes” and 30% said “no.”
In North America, the majority of participants (60%) answered “yes,” 30% answered “no,” and the remaining 10% answered “couldn’t remember.” When separated by the medicine forms, the percentages differed. For those using bottles but not blister packs, 51% said “yes,” 37% said “no,” and 11% answered “didn’t remember.” For respondents using blister packs and not bottles, 75% said “yes,” 20% said “no,” and 5% answered “didn’t remember.” For those using syringes and not blister packs or bottles, 48% said “yes,” 35% said “no,” and 17% answered “couldn’t remember.”
Taking Medicine on Schedule
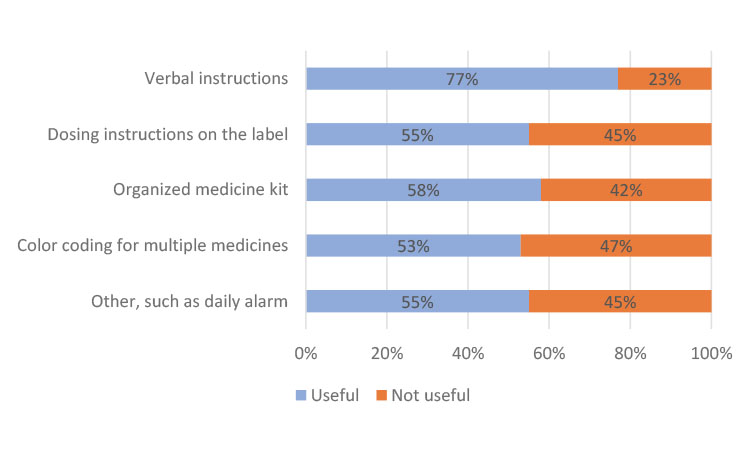
To better understand any issues with taking medications on schedule, the participants were asked what would help remind them to take their clinical trial medication. They were asked to rate several options on a scale from 1 to 4, where 1 indicated “not at all useful” and 4 indicated “very useful.” In other translations of the survey response options, “useful” was replaced with “helpful.”
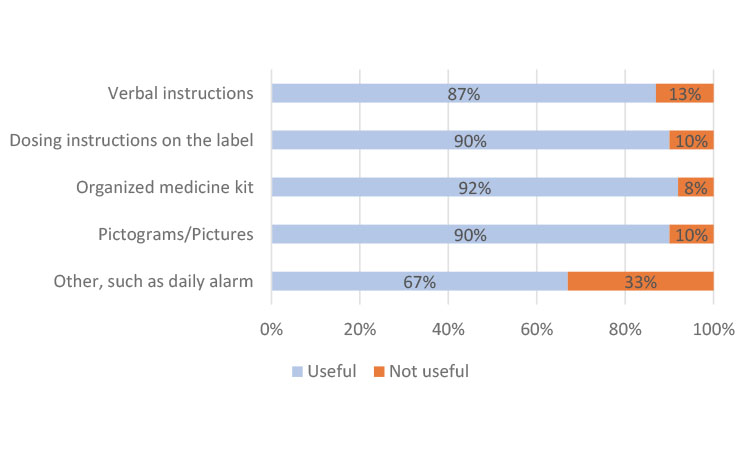
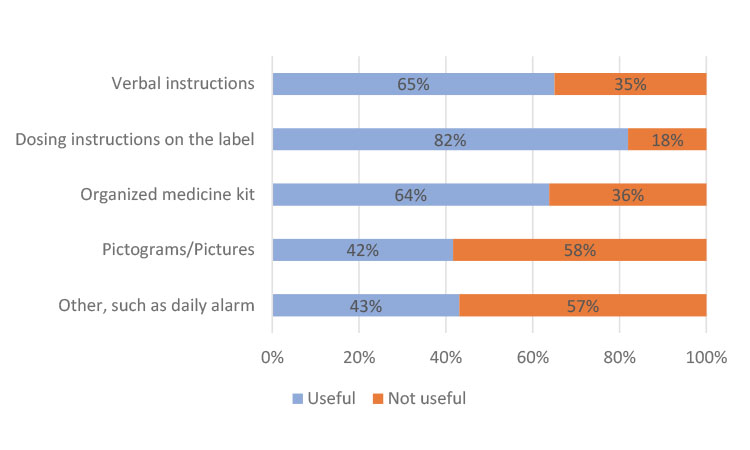
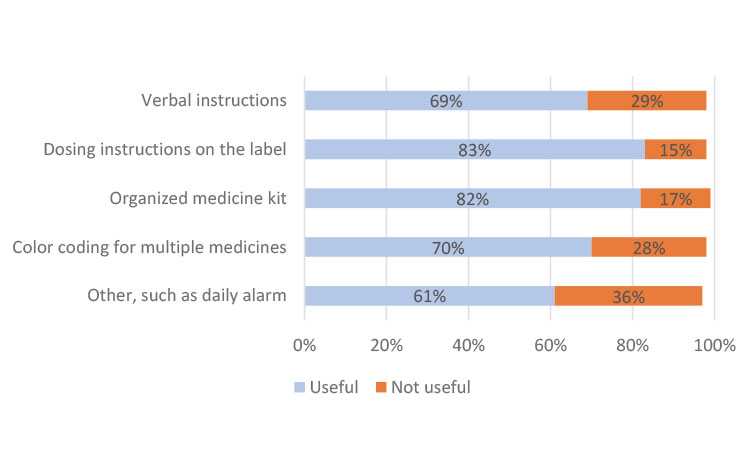
In China, the participants preferred instructions from their clinician at every visit and only indicated a slight preference in helpfulness in the other categories (Figure 3a). In Japan, most participants found all methods useful but cited individually organized daily or weekly dosing units in the kit as the most helpful (Figure 3b), which could be attributed to the high use of blister packs. The participants from Europe indicated a strong preference for dosing instructions on the label (Figure 3c), similar to results from participants in North America (Figure 3d).
Evaluating the results on a global scale, “dosing instructions on the label” (73%) and “verbal instructions from my physician/nurse/pharmacist” (69%) were cited as the two most useful mechanisms to help participants take their clinical trial medication. Using an organized medicine kit, such as trial medication organized in individual daily or weekly dosing units, was also cited as important or very important by 66% of global respondents.
Most Helpful Form of Instruction
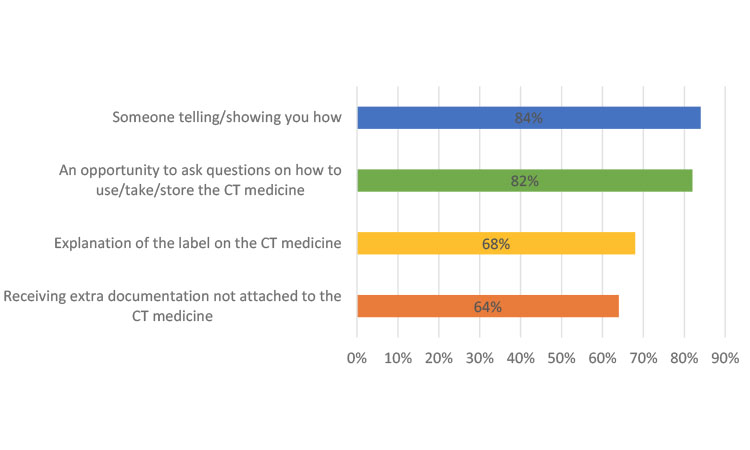
Survey participants were asked to think about how they “learned to use, take, and store the clinical trial medicine,” and rate the helpfulness of various methods. Combined results from Europe, North America, and China show a slight preference (84%) for “someone showing/telling you” how to take clinical trial medication as compared with “an opportunity to ask questions” (82%). Slightly less preferred methods were to receive an “explanation of the label” (68%) and “receive extra documentation” (64%) (Figure 4). These data confirm the ongoing need for person-to-person explanations, which can be supplemented by printed material.
Packaging Preferences
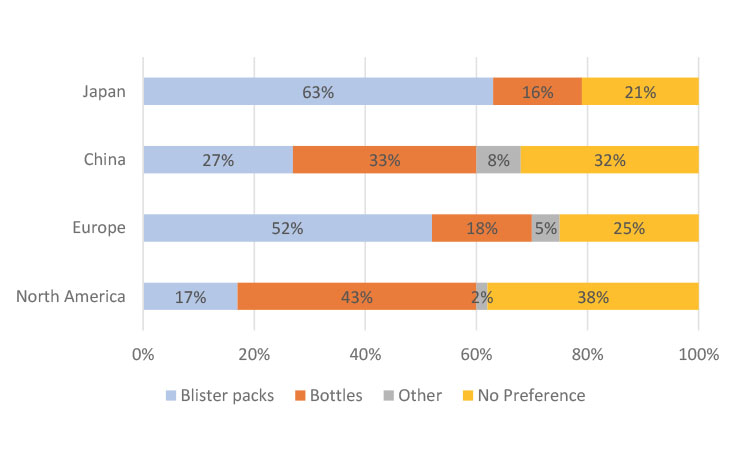
Respondents in Japan and Europe preferred to receive their medications in blister packs, whereas respondents in North America preferred bottles. Respondents in China had a relatively equal distribution between the different kinds of packaging.
The packaging preferences tended to align with the packaging use reported in the clinical trial in which the respondent participated (Figure 5). For example, 71% of survey respondents from Japan were using blister packs and 14% were using bottles in their clinical trial. Of the group that was only using blister packs in their clinical trial (n = 878), 71% preferred blister packs, 22% had no preference, and only 7% preferred bottles. For the respondents only using bottles (n = 134), 66% preferred bottles, 17% preferred blister packs, and 17% had no preference.
Of the survey respondents in Europe who used blister packs (37%), 70% preferred blister packs in their clinical trial and 23% specified no preference. Only 3% of blister pack users in Europe preferred bottles. In the North American survey, of the 482 respondents who only used bottles, 59% preferred bottles, 8% preferred blister packs, and 33% had no preference. However, of the 339 respondents who only used blister packs, 38% preferred bottles, 34% preferred blister packs, and 27% had no preference. For respondents who used both bottles and blister packs in their clinical studies, 48% preferred bottles, 25% preferred blister packs, and 28% had no preference.
In a related question asked only in North America, Europe, and China, participants were asked if they kept their medicine in its original container. This has been a concern in the industry because patients may remove their medication from the clinical trial kit provided, thus risking incorrect dosing. However, participants in these three regional studies reported similarly encouraging responses: 86% of participants in Europe, 84% of participants in China, and 86% of participants in North America kept their medicines in the original container.
Most Important Characteristics of an IMP
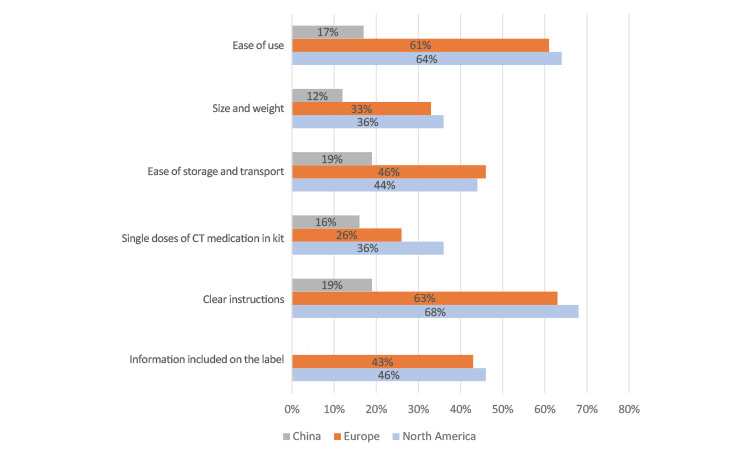
Survey participants were asked, “How important is each of the following medicine kit characteristics to you when thinking about its effect on your overall experience in your clinical trial?” and “Can you indicate the importance of each characteristic on a scale of 1 (not at all important) to 4 (very important).” In North America and Europe, participants overwhelmingly rated “ease of use” and “clear instructions” as the most important characteristics of their IMP kits (Figure 6a). In China, participants did not indicate a strong preference for IMP characteristics as compared to North America and Europe.
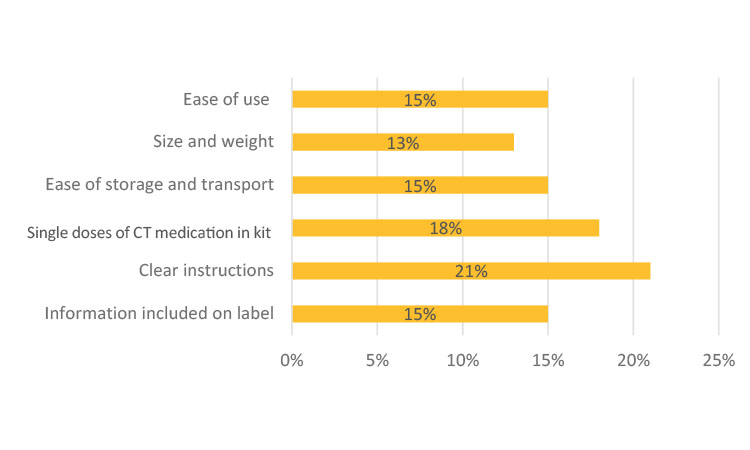
In Japan (Figure 6b), this question was translated to be better understood by the participants as “Would you like to request that sponsor companies improve the following areas?” The participants selected their desired level from 4 (“very much”) to 1 (“not at all—meets expectations”).
Reuse and Return Behaviors
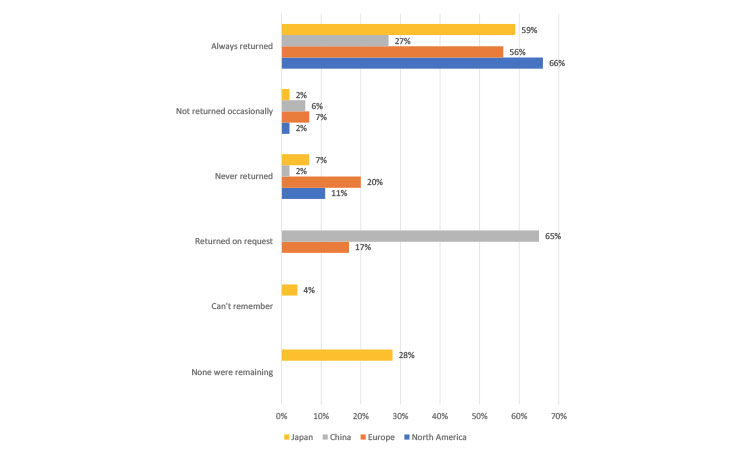
Participants were asked if they returned their used and unused clinical trial medicine to their medical center. The results across Europe, China, and Japan were consistent with the results in the original North America study, which found that an unacceptable percentage of participants did not return unused medication to the clinical sites. This is a result that the industry needs to mitigate against globally (Figure 7).
The high percentage of “returned on request” results for the China study may reflect the participants’ interpretation of the question and represent those participants that returned supplies as they were “requested” to do so by the clinical site. These ¬ findings may also highlight that in-person communication is important and that patients may require explicit requests from their clinical site to return unused medication.
In Japan, participants could also indicate additional choices like “can’t remember” and “none were remaining” when describing their return behaviors. In China and Europe, participants could select “returned on request.”
Supplementing this question, the survey team in Japan also asked the group of participants who answered, “not returned occasionally” or “never returned” (9% of total surveyed population), why they kept unused clinical trial medicine. In this 9% of the total survey population, 34% of these participants said that the sites “never asked for a return,” 32% said they had no visit scheduled to return the medication, 22% forgot to return it, and 12% said they wanted to keep unused medication for future use if they received the same diagnosis.
Pictograms and Booklets
The researchers wanted to understand if the booklet labels and pictograms serve as an effective way to communicate medical information to patients. These studies were also intended to help evaluate booklet labels, an area of intense focus in clinical trial design.
In Europe, 75% of survey participants reported that they had not seen pictograms on their kit but 41% found that text and pictograms together were helpful. Regardless of whether they had seen pictograms on their medication, nearly all the participants from Europe were able to identify four common pictograms correctly.
In China, 82% of respondents found the pictograms at least “somewhat helpful.” These data correspond to the original 2013 survey in North America, in which most survey participants found the same pictograms “helpful.” In Japan, survey participants were given slightly different questions. They were asked if they opened and read the booklet label. Also, 65% said they had opened and read the label of each container at least once, whereas 21% had done so on some, but not all, containers. In this survey, 7.1% said they never opened or read the booklet label and 6.5% reported reading the booklet label every time.
In comparison to pictograms, the booklet label seemed to have limited use to participants. Half of the survey participants in Europe said they had never opened or read the booklet label. In China, 55% said they relied on instructions from the booklet label; however, 17% said they never opened their booklet labels. Although these data showed a geographic difference, the results indicate that patients frequently prefer and rely on verbal information from the clinical site rather than booklet labels.
Survey participants who did read their booklet labels found it easy to find their language and read the information; most participants in the Europe survey found that the text size was large enough to read. In Japan, survey respondents were asked about how easy it was to find their language of choice in the booklet label. Further, 83% reported that it was “very easy” or “somewhat easy” and 11% reported it was “somewhat difficult “or “very difficult,” whereas 6% reported “could not remember.”
Home Delivery
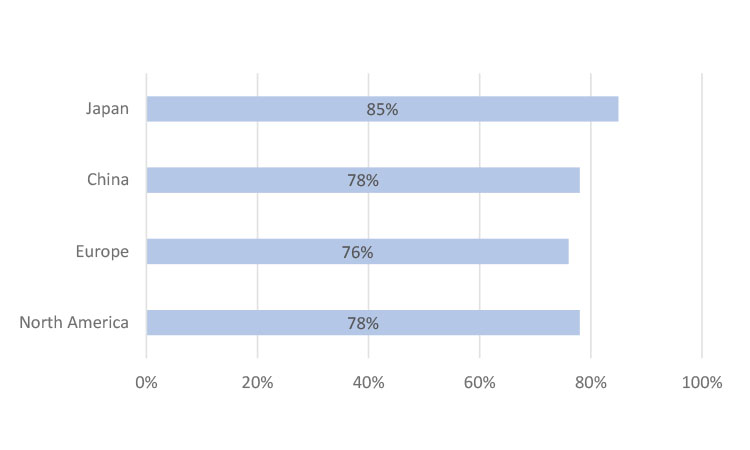
Patients often have to travel long distances to participate in studies. To improve patient recruitment and retention, some sponsors are considering ways in the future to send IMPs directly to patients’ homes to help ease participant burden. The survey team wanted to gauge patients’ future preferences for this. Participants were asked, “If it was possible to have repeat prescriptions or refills of your clinical trial medicine delivered to your home, how helpful would you find this?” More than 75% of respondents in North America, Europe, and China reported that having IMPs delivered directly to their homes would be helpful; in Japan, it was 85% (Figure 9).
Medicine Kit Size
From the outset of this work, size, storage, and ease of transportation of IMP kits were expected to be of concern to patients. Survey participants in North America, Europe, and China were asked about their thoughts on the size of the medicine kit as it concerned transportation and its ease of storage at home. Most of the respondents in these three regions (>80%) said their IMP kit was “very easy” or “somewhat easy” to store. Similarly, more than 70% of global participants said their medicine kit was easy to transport based on its size. Although the same question was not directly explored in Japan, as shown in Figure 6b, only 13% of participants in Japan expected improvement on size and weight for their medication kits. Thus, from the survey results, it was therefore surprising, but reassuring, that there appeared to be general satisfaction observed in these regions regarding kit size and weight for storage and transportation purposes.
Information Delivery Preferences
To gauge patients’ preferences for the way they would like to receive additional information, participants were asked, “In addition to receiving information from your healthcare worker, tell us how useful would it be to receive information in the following ways?” They could rate the usefulness of various communication methods in a clinical trial.
Participants in most regions indicated a strong preference for email, followed by text messages (Table C). It is interesting to note, however, that email was the most preferred method in Europe, North America, and Japan, but least preferred in China, potentially because email is not significantly used as a daily or instant electronic communication tool in China.
To gauge interest levels of using other communication methods as reminders, participants in China were asked, “How interested would you be in receiving an electronic device along with your clinical trial medicine to remind you to take your medicine and document that you have taken your medicine?” and 66% of the participants said they would be “very interested” or “somewhat interested” in an electronic device as a reminder system.
In Europe, participants were asked a related question on communication: “How interested would you be in receiving electronic or telephone reminders each time you need to take your clinical trial medicine?” and 44% said they would be “very interested” or “somewhat interested” in such reminders.
Discussion and Key Findings
Retention and compliance of clinical trial participants is crucial to drug development research, but IMP professionals, as well as study sponsors and suppliers, do not interact directly with participants and may be unaware of the patient experience as it relates to using trial medications. This series of four regional studies aimed to compare specific clinical trial preferences, help inform industry about these patient preferences, and ultimately develop global guidelines for patient-centric design of IMPs and create best practices for communicating their proper use.
Ease of Use
One of the most important characteristics of a medicine kit cited by participants was ease of use. The original study in North America suggested a high level of satisfaction with the ease of use of IMPs, which was also reflected in the expanded surveys in Europe, China, and Japan. These results could suggest that our industry is adequately meeting the needs of patients. However, given that ease of use was reported as a highly valued quality of a medicine kit, sponsors and suppliers must continue to ensure their IMPs support patient compliance efforts by meeting end users’ needs. Clear instructions were also cited as an important characteristic in an IMP, emphasizing the essential role of in-person communication with clinical research staff to verbally explain the use of the medication.
| Region | Top 2 Delivery Methods Preferred |
|---|---|
| Japan | 1. Email 2. Postal mail |
| China | 1. Text 2. Postal mail |
| Europe | 1. Email 2. Text |
| North America | 1. Email 2. Text |
The Role of Kit Design
The kit design plays a role in supporting taking medicine on schedule, but there are strong regional differences. Depending on the type of packaging, such as blister packs, bottles, and syringes, the design may also play a role to support taking medication on schedule. Other methods, like organizing medicine kits in daily or weekly dosing units or color-coding, might help supplement healthcare workers’ efforts to ensure adherence to medication schedules. With the exception of the results from respondents in Japan, where there was a high percentage of blister pack users, all other regions indicated that kit design did not strongly support taking medicines on schedule, citing this as an area for potential improvement to strengthen compliance.
Primary Communication Methods
Maintaining compliance with the study protocol relies on strong communication with participants. Dosing instructions on the label and verbal instructions provided at the site or with a pharmacist are still considered very useful to global study participants.
Written Communication
Booklet design is an important issue for regulators, who are concerned that patients do not read booklet labels; a concern expressed by some is that medicine kits are often returned with unopened booklets. Although a majority of respondents in Japan reported reading the booklets, nearly half of the respondents in China and Europe said they never opened or read the booklet label. A potential explanation for this result could be that some clinical sites are obliged, for a variety of reasons, to add their own study label to IMPs; this could be the label that patients read and remember. Clinical trial stakeholders should keep these findings in mind and not rely on patients to read the booklet, but rather ensure that comprehensive verbal communication is employed at the time of a study visit.
Pictorial Communication
Pictograms serve as another vehicle for communication, especially regarding storage information. In these studies, nearly all participants from Europe were able to identify four common storage-related pictograms correctly. A majority of participants from China found the pictograms at least somewhat helpful. These data correspond to the original 2013 survey in North America, in which most survey participants found the same pictograms helpful.
Electronic Communication
In terms of strengthening ongoing communication with clinical trial participants, email and text message were listed as preferred methods after face-to-face communication in the clinic. Although data on reminder preferences were not collected in Japan or North America, the participants in China and Europe indicated some level of interest for electronic or telephone reminders to take their medication. Because adoption of mobile technologies has grown over the past few years, the preference for electronic reminders may change and should be further explored among clinical study participants.
Returning Unused Medications
It is assumed that all clinical site staff communicate the need for the timely return of all unused medications. The majority of participants across all four surveys report either returning or using all the medication. However, a concerning percentage of participants reported the intent of retaining or using unused and/or unreturned medication. Clinical trial stakeholders must determine how best to recover or account for all unused medications.
Delivery Methods
In considering how to ease the burden on patients in obtaining medication refills, home delivery was of interest across all four regions. In North America, this was a particular wish of younger participants, who may be short on time. In this arm of the survey, elderly participants placed a strong value on visiting the clinical site and having an opportunity to receive medication and information directly from the study staff or a pharmacist. Age was not collected in the China or Japan surveys, but it would be a valuable data point to collect in any future survey to understand whether this sentiment aligns with North American participants.
Conclusion
In ongoing efforts to incorporate patient-centric practices into clinical research, study sponsors, IMP suppliers, and clinical sites need to consider how to best support patient retention and compliance by evaluating the patients’ overall ease of use with the medication and by facilitating clear, consistent communication regarding instructions.
Regardless of the medication packaging, the instructional label, face-to-face explanations about the usage, and return of medication are essential to support clinical trial participants. Compliance may also be boosted by incorporating secondary measures, such as sending reminders through email or text messages. These methods of communication may not be widely employed, but would be accepted by study participants if employed in a secure manner through the clinical site. Finally, participants find their current medications easy to transport and store, but would also welcome home delivery of their medications.
Ever-improving, modern-day communications will facilitate a greater dialogue between patients and IMP professionals. The production of patients’ clinical medication supplies can function as a two-way process by first accessing the needs and preferences of the trial patient population and then determining the best design and delivery method. Ensuring that communication continues throughout the trial to accommodate any learning and modifications needed to assist the patient will also improve retention and compliance. Coupled with new advances in clinical supply manufacturing methods, the industry can expect shorter lead times to prepare and deliver medications, enabling more flexibility in the clinical supply chain.
The implementation of direct-to-patient (DTP) supply models may also improve patient recruitment and adherence to medications. Although this more-user-friendly approach offers many benefits to study participants and clinical research sites, employing a DTP model also requires a well-coordinated effort between regulators, legal teams, clinicians, and logistics providers. Supporting a patient-centric supply chain and balancing cost considerations is not an easy feat. With these global survey results, the ISPE team and its supporting sponsors hope that this article will create greater awareness about patients’ current usage and encourage stakeholders to evaluate the specific needs of patients as they relate to their study. Using this article as a guide, stakeholders can implement best practices in the design of IMPs and communication of their use to ensure greater patient safety, increase compliance in their studies, and create more consistent data in clinical trials.
Further Reading
- Pharmaceutical Research and Manufacturers Association and Battelle Technology Partnership Practice. “Biopharmaceutical Industry-Sponsored Clinical Trials: Impact on State Economies.” March 2015. http://phrma-docs.phrma.org/sites/default/files/pdf/biopharmaceutical-industry-sponsored-clinical-trials-impact-on-state-economies.pdf.
- Sadler-Williams, Esther. “Patient Perceptions of IMPs. ”Pharmaceutical Engineering” 36, no. 1 (January/February 2016): 22–http://www.ispe-pcc.org
Acknowledgments
The authors would like to acknowledge and thank Catalent Pharma Solutions for providing technical support in the final compilation of this article and for promoting the findings of our research. The patient survey was supported by ISPE and sponsored by several organizations: Almac, Boehringer, Ingelheim, Cardinal Health, Catalent Pharma Solutions, Cenduit, Eli Lilly and Company, Fisher Clinical Services, Merck & Co., Inc., Novartis, Novo Nordisk, PAREXEL, Pfizer, and UCB.
North America and Europe team members: Christine Milligan, previously Catalent; Karleen Kos, ISPE; Michelle Foust, Almac; Karen Gram, Novo Nordisk; Kunal Jaiswal, Catalent Pharma Solutions;
Esther Sadler-Williams, SimplyESW; Kristen DeVito, Catalent; Julian Schultz, Takeda; Ken Getz, CISCRP; Annick Anderson, CISCRP; Nova Getz, CISCRP; Chedia Abdelkafi , UCB; Petra Bielmeier, F. Hoffmann-La Roche AG; Samantha Carmichael, NHS: Greater Glasgow and Clyde; Mike Davis, GlaxoSmithKline; Massimo Eli, Merck & Co., Inc.; Marianne Oth, Eli Lilly and Company; Paula McSkimming, Roberson Centre for Biostatistics, University of Glasgow; Anthea Mould, NIHR Clinical Research Network, NHS UK; Sue Pavitt, EUPATI; and LaTanya Benford, ISPE.
China team members: Lynn Wang, Merck & Co. Inc.; Tracy Han, Ferring Pharmaceuticals Inc.; Shuting Li, GCP Office, Cancer Hospital Chinese Academy of Medical Science; and Hong Fang, GCP Office, Cancer Hospital Chinese Academy of Medical Science. Japan team members: Chie Igushi, Pfizer; Hiroaki Ishii, Mitsui Soko; Kaoru Ide, Toray; Akihiro Shiga, UCB Japan; Chiaki Yamauchi, Nippon Boehringer Ingelheim; Hirofumi Suzuki, Bayer; and Robert Kamphuis, Pfizer.
ISPE administered the survey and aggregated the responses; NHS, NIHR, and EUPATI disseminated the surveys to patients through clinical trials pharmacies, research nurses, or patient advocacy groups; the Robertson Centre for Biostatistics, University of Glasgow, analyzed and reported on the resulting data.
The Europe team partnered with three agencies that had access to patient groups: UK National Health Service (NHS), UK National Institute for Health Research (NIHR), and European Patients Academy on Therapeutic Innovation (EUPATI). In China, the ISPE China IP CoP partnered with Drug Information Association China to enlist five site management organizations to collect responses: Hangzhou Tigermed Consulting Co., Ltd.; LinkStart; Medkey Med-Tech Development Co. Ltd.; SMO ClinPlus; and WuXi Apptech. In Japan, the ISPE Japan IP CoP enlisted cooperation of the professor to collect responses: Professor Kaori Muto of the Institute of Medical Science, The University of Tokyo


