CAPA Maturity

The ISPE Advancing Pharmaceutical Quality (APQ) team is developing a framework by which a company can assess its maturity in relation to quality culture, operational excellence, and ICH Q10 elements, using the CAPA system as the focus of the pilot.
Corrective and preventive actions (CAPAs) are indicators of company health. They demonstrate whether issues are acknowledged, tracked and, ultimately, remedied in an effective and permanent manner.
The timeliness and robustness of these records also indicate whether a company demonstrates effective planning and/or has sufficient resources to manage and resolve past and potential issues. In this way, the effectiveness of a company’s CAPA program also has a relationship with other key indicators of company health including, but not limited to, management responsibilities.
The ISPE Advancing Pharmaceutical Quality (APQ) team is developing a framework by which a company can assess its maturity in relation to quality culture, operational excellence, and ICH Q10 elements, using the CAPA system as the focus of the pilot. As a primary tool leveraged for tracking and resolving issues, a robust CAPA program is designed to identify signals and improvement opportunities from multiple inputs (Figure 1). Additionally, a robust CAPA system can strengthen the performance and efficiency of other areas of the business as demonstrated through studies such as the University of St. Gallen work with FDA.1
- 1Friedli, T., S. Koehler, P. Buess, P. Basu, and N. Calnan. “FDA Quality Metrics Research, Final Report.” July 2017. www.tectem.ch
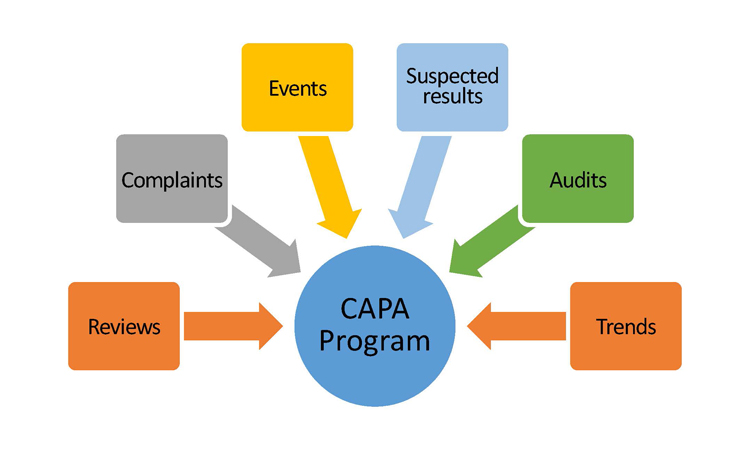
The program being developed will:
- Collect signals from your CAPA system to identify issues that indicate a need for improvement.
- Identify the root causes of issues to enable an appropriate remediation.
- Implement corrective actions to eliminate those root causes and prevent recurrence.
- Implement preventive actions to eliminate potential root causes to prevent future occurrence.
This program will consist of three parts:
- Identify current maturity levels against a number of CAPA elements (e.g. root cause analysis, CAPA effectiveness, governance, and management oversight).
- Suggest tools to help improve each area.
- Track KPI measurements in each area as needed.
Assessment results will be presented in a table format similar to a “heat map” that indicates a company’s place on the maturity continuum (Figure 2). It will help users assess and prioritize which areas of the CAPA program will yield the best return for their improvement efforts.
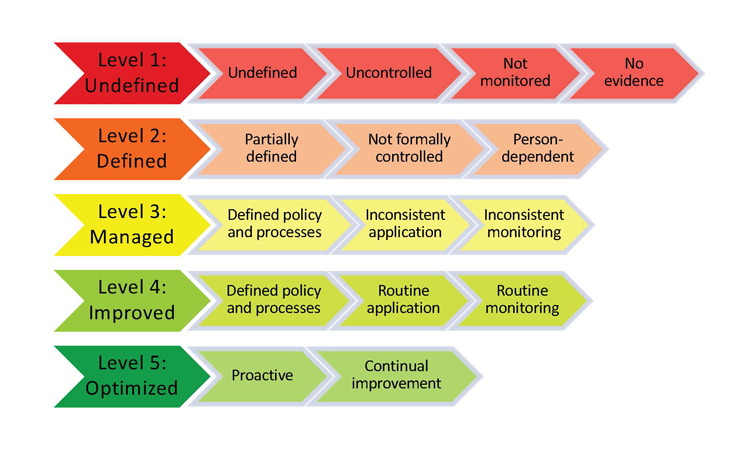
Once a company determines where they fall on the maturity continuum, they may leverage suggested improvement tools to increase performance, then track it by using the metrics integral to each element.
For example, if a company were to measure effectiveness of the CAPA element “root cause analysis” and identify deficiencies in that area, they may choose to utilize the “5 Why” fishbone diagrams or other tools to improve performance. They may then choose to measure their success using a metric that tracks the effectiveness of their CAPA remediation efforts and/or repeat CAPAs.
As each focus area improves, companies may choose to reprioritize CAPA elements and/or modify each performance target related to existing metrics until they have reached their target level of CAPA maturity.
As the APQ Team continues to develop the assessment program, learnings from the CAPA pilot will be built into the overall assessment suite for quality culture, operational excellence, and ICH Q10 elements to assist companies in their assessment and improvement journey.


