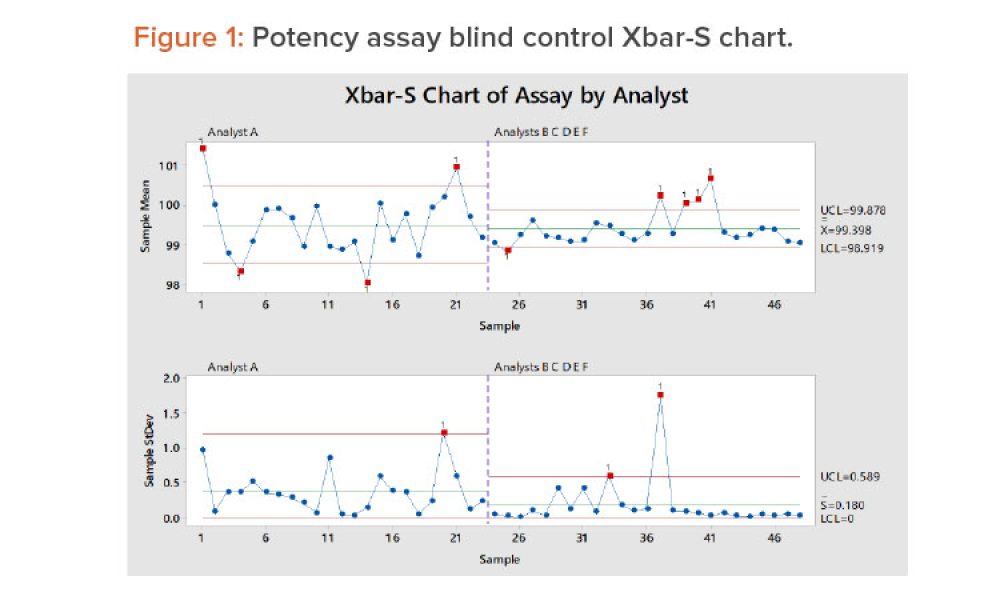
Good data are a characteristic of good science. Quality data are arguably more important today than ever before and are considered by many to be a corporate asset because they are used to develop products and processes, control our manufacturing processes, and improve products and processes when needed.1
- 1Snee, R. D. “Crucial Considerations in Monitoring Process Performance and...