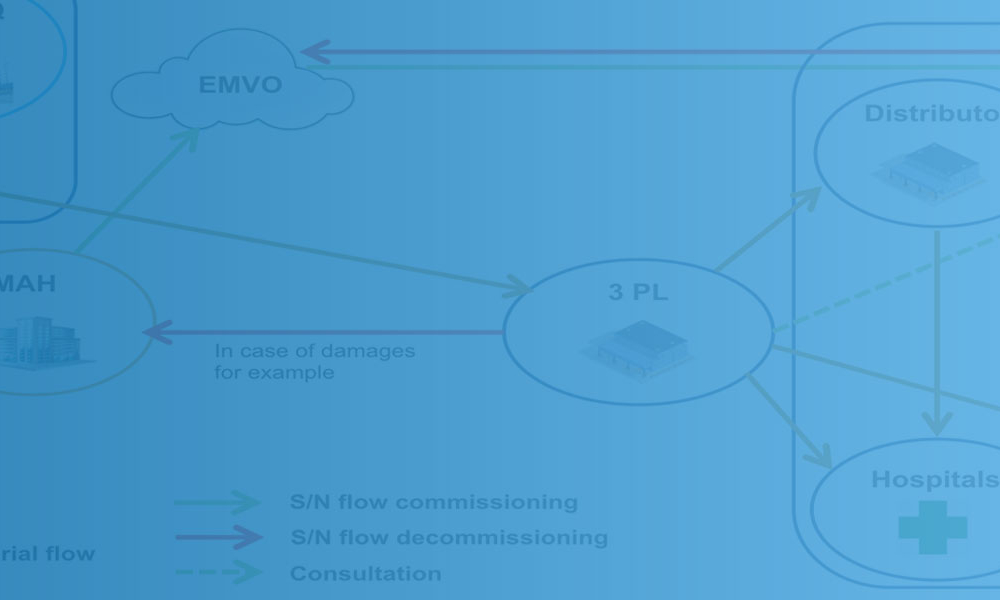
Features
To meet the EU serialization deadline on 9 February 2019, pharmaceutical companies and their contractors have had to reorganize their manufacturing lines and logistics to ensure compliance with the EU’s Falsified Medicines Directive (FMD) of 2011 and the EU Commission Delegated Regulation 2016/161 of 2016. Worldwide, other anticounterfeiting regulations are already in place or coming soon in...