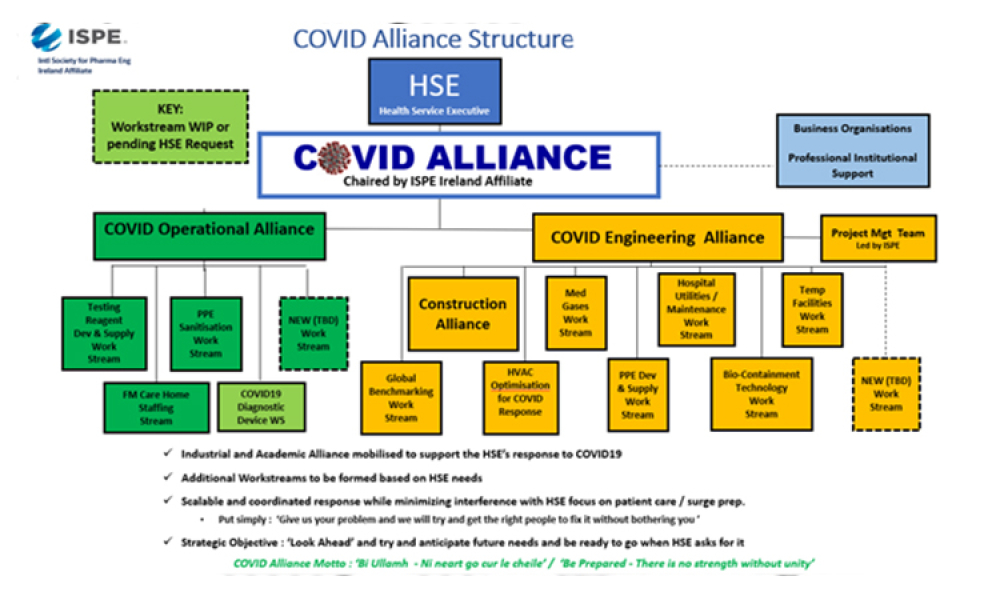
Early in March 2020, many around the world viewed the sight of collapsing intensive care units in Northern Italy, overwhelmed by COVID19, with great anxiety and we wondered how we might address the inevitable arrival of the virus at our doorsteps. This concern led to a home-grown initiative in Ireland that brought together representatives of health, commercial and academic organisations...
COVID-19
Information on COVID-19
The current COVID-19 pandemic has heightened the awareness and attention to virus safety and risk mitigation for manufacturers. Each manufacturer has a strategy in place to mitigate the risk, however by manufacturers working together, this can lead to synergies and best practices in the way that the risk is managed. Also, additional guidance is required by the Industry in a field that is...
At the ISPE Singapore Affiliate’s 20th Anniversary Conference on 9-12 December 2020, four international regulators discussed how regulatory authorities and industry were responding to the COVID-19 pandemic crisis.
FDA Commissioner Stephen M. Hahn, MD, spoke about the lessons learned from COVID-19 in a presentation during the ISPE Annual Member Meeting on 5 November at the 2020 ISPE Annual Meeting & Expo. He discussed how the FDA has been responding to the pandemic, including how actions taken in response to the pandemic have been combined with FDA knowledge from prior public health crises to...
Vaccine development is an intricate undertaking, which may involve numerous challenges from the initial process of identifying an antigen to the final steps of delivering and administering the licensed product. The COVID-19 pandemic has put a spotlight on the science of vaccine development. As the world awaits a vaccine for the coronavirus, manufacturers face unprecedented pressure to respond...
This article updates a 2006 Pharmaceutical Engineering® Online Exclusive article titled “Avian Flu—Is My Company Prepared?” by Wendy Haines and Martin Rock.