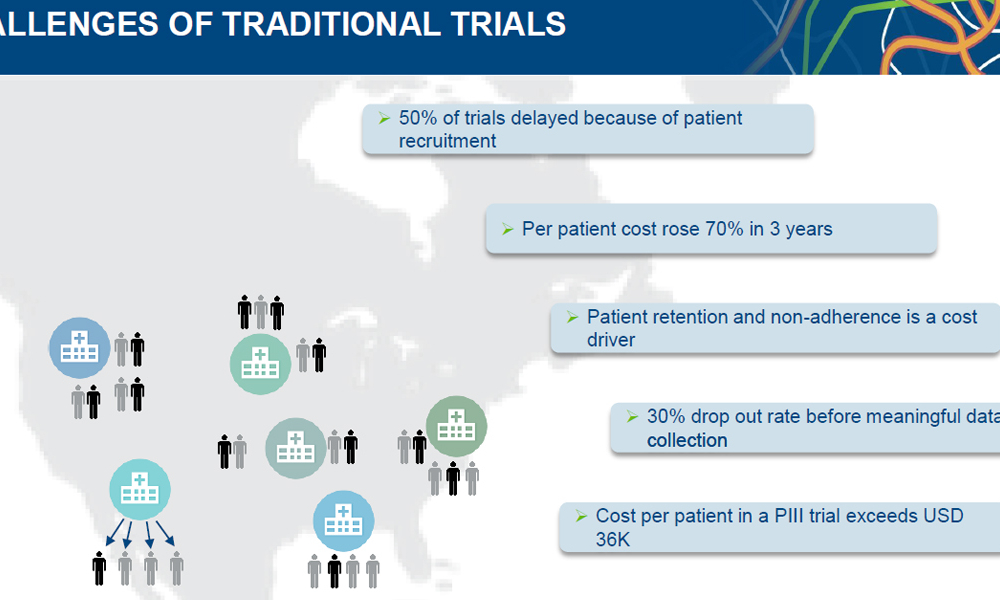
Being on the cutting edge of drug development is the goal of most pharmaceutical companies, but a new drug won’t work if the patient doesn’t take it. It’s a vexing problem that developers of healthcare technologies hope to address.
Patient-Centric Logistics
Features
Features
This article examines patient preferences in one facet of clinical research: the experience related to the use of investigational medicinal products (IMPs). As patients have become more involved and informed in their healthcare choices, the “voice of the patient” has been increasingly incorporated into the drug development process. Given that the success of a clinical study relies on the...
Insights
Features
A Danish pharma company's strong customer focus and determined digital drive have important lessons for other businesses.