Single-Use Technology
iSpeak Blog
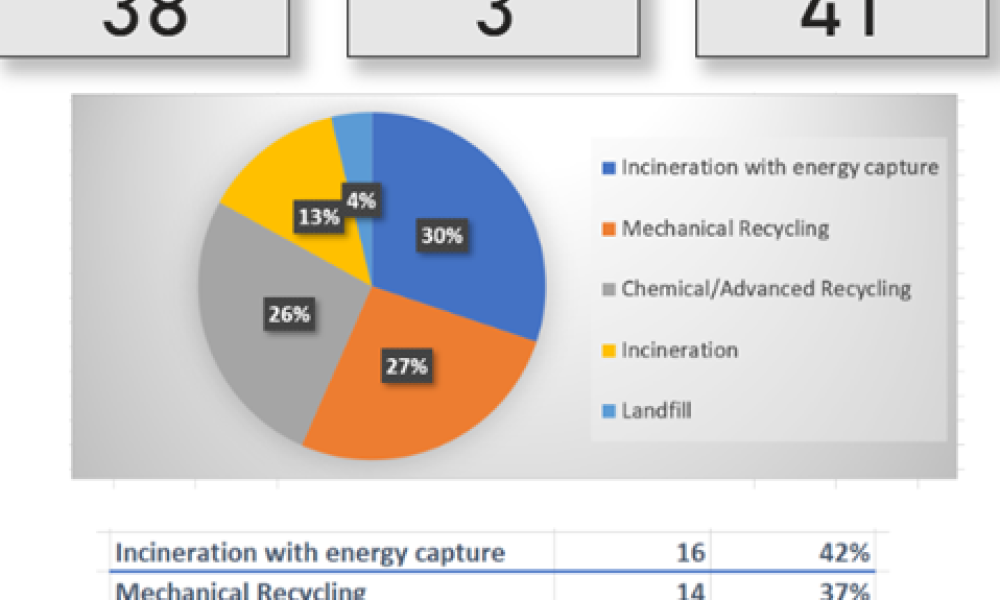
In the iSpeak Blog posting on 14 March 2023, the first part of this blog series introduced the results of a survey that was conducted of users of single-use assemblies. The focus...