 ISPE’s Advancing Pharmaceutical Quality™ (APQ) program is an industry-led quality management maturity assessment and benchmarking program that provides a practical set of tools and systematic approaches for organizations to advance the effectiveness of their PQS. The program is aligned with international initiatives that promote quality excellence, as well as with the FDA’s focus on quality management maturity and rating the maturity of manufacturing facilities.
ISPE’s Advancing Pharmaceutical Quality™ (APQ) program is an industry-led quality management maturity assessment and benchmarking program that provides a practical set of tools and systematic approaches for organizations to advance the effectiveness of their PQS. The program is aligned with international initiatives that promote quality excellence, as well as with the FDA’s focus on quality management maturity and rating the maturity of manufacturing facilities.
ISPE’s APQ Program:
- Recognizes that the ability to advance quality management maturity lies within the industry itself (developed by industry representatives for use by industry)
- Builds upon the ICH Q10 model and enhances the PQS elements with the aspects of cultural excellence, operational excellence (OPEX), knowledge management, and continual improvement
- Provides a comprehensive approach for assessing and improving an organization’s quality management maturity to advance the state of quality within the organization
APQ Framework Overview
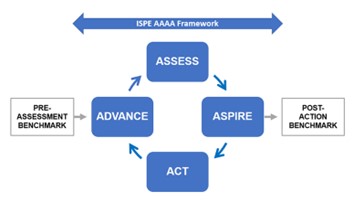
At the core of the APQ Program is the Assess, Aspire, Act and Advance framework. Each APQ Guide contains quantitative and qualitative tools to assess the organization’s current state of quality, diagnose gaps, and identify improvement targets – Assess and Aspire. The Guides explain how to use this information to develop an Improvement Action Plan that the organization can execute and evaluate for effectiveness – Act and Advance.
The APQ Program was developed by ISPE members and evolved from the ISPE Quality Metrics pilot programs, representing extensive industry engagement, collaboration with academia and other associations, and knowledge sharing with regulatory agencies.
APQ Guide Series
The program consists of five Good Practice Guides: one for each of the four elements of an ICH Q10 Pharmaceutical Quality System plus Cultural Excellence, bookended by an optional benchmarking tool developed by University of St. Gallen.
The APQ Guides series proves a set of tool and systematic approaches – the Assess, Aspire, Act, and Advance Framework – for organizations to advance the maturity and effectiveness of these PQS elements.
APQ Training Course
The ISPE Advancing Pharmaceutical Quality (APQ) Program has been developed by industry representatives, for industry use, to provide a practical framework that organizations can use to assess and advance the state of quality within their organization. The APQ program recognizes that the ability to advance the maturity of quality management lies within the industry itself and provides a range of sustainable and practical quality management improvement strategies.
In this training, you will be led through the APQ guide series for Quality Management Maturity including how to leverage structured tools using a tested industry best-practice approach to coordinating, conducting, presenting, and reporting out on assessment outcomes. You will learn how the APQ methodology is used to improve the current state of quality and how it can benefit your organization through the Assess, Aspire, Act, Advance Framework.
Articles and Blogs
Articles
- Elevating Pharmaceutical Quality: FDA’s Office of Quality Surveillance and ISPE’s APQ™ Program
- ISPE Quality Management Maturity Program: Advancing Pharmaceutical Quality
- ISPE’s Advancing Pharmaceutical Quality Program Progresses
- ISPE’s APQ Program & Guides Advance Pharmaceutical Quality
- FDA’s Approach to Advancing Quality Surveillance: PQS Effectiveness, Post-Market Quality Surveillance, Quality Metrics and Quality Management Maturity
- ISPE Advancing Pharmaceutical Quality Program Complete
- Quality Management Maturity Framework - ISPE Advancing Pharmaceutical Quality Team and University of St.Gallen OPEX
Webinars
- Advancing Pharmaceutical Quality, Change Management
- Advancing Pharmaceutical Quality, Corrective Action/Preventive Action (CAPA) Webinar
Related Publications
.png) Cultural Excellence Report
Cultural Excellence Report
Examining the powerful force that culture exerts on day-to-day operations within organizations, ISPE’s Quality Culture Team has established that although for many the concept of quality culture remains abstract, the behavioral impacts are very real indeed. The Cultural Excellence Report contains a collection of practical, powerful tools and a comprehensive behavior-based approach for improving quality culture as a means of delivering enhanced quality outcomes.
 Guide to Improving Quality Culture in Pharmaceutical Manufacturing Facilities: Root Cause Analysis
Guide to Improving Quality Culture in Pharmaceutical Manufacturing Facilities: Root Cause Analysis
Published: 2019
Pages: 10
Quality Culture has always been important within pharmaceutical manufacturing operations. Strong companies know this and have invested resources in systems and personnel to support and promote a focus on quality processes, product quality, and meeting patient needs. More recently, health authorities have placed additional emphasis on quality culture by including it in guidance documents and inspection protocols such as PIC/S Data Integrity Guidance , FDA New Inspection Protocol Project (NIPP) , and MHRA Data Integrity Guideline.
ISPE and the Parenteral Drug Association (PDA) have jointly developed this guide which identifies specific aspects of quality systems and culture and recommendations for tools, techniques, and processes.
ISPE continues to support quality metrics and quality management maturity programs that have value to regulators, industry and patients. ISPE’s input is experiential and data driven, drawn from:
- A quality metrics initiative initiated in 2013 by a core team and senior leaders representing more than 10 companies
- An extensive pilot program conducted in cooperation with McKinsey and Company with participation from 28 companies and 83 sites. These companies and sites represented a wide range of technologies and included contract manufacturing organizations (CMOs) and laboratories, and drug substance manufacturing sites.
- Ongoing robust dialog with pilot companies and the industry at large at ISPE conferences, workshops, and forums
Insights gained over the past nine years remain consistent:
- Definitions and terminology must be exact in order to avoid multiple interpretations by participants, and to enable consistency in reporting and ability to conduct analysis
- Many companies currently collect metric data at a site level and often utilize definitions different from the FDA or agreed ISPE harmonized definitions. Moreover, there are often different definitions or variations in interpretations of a definition between sites in the same company. Consequently, there is a significant burden for companies to collect data against harmonized definitions.
- Frequency of data collection (e.g., quarterly, annually) has a major impact; analytic and reporting burden is proportional to the number of data points collected
- Producing metric data on a product basis is difficult for products manufactured with complex supply chains and for companies with a large number of products. Historically, these data were not commonly produced or included in periodic product reviews (PPRs).



.jpg)



