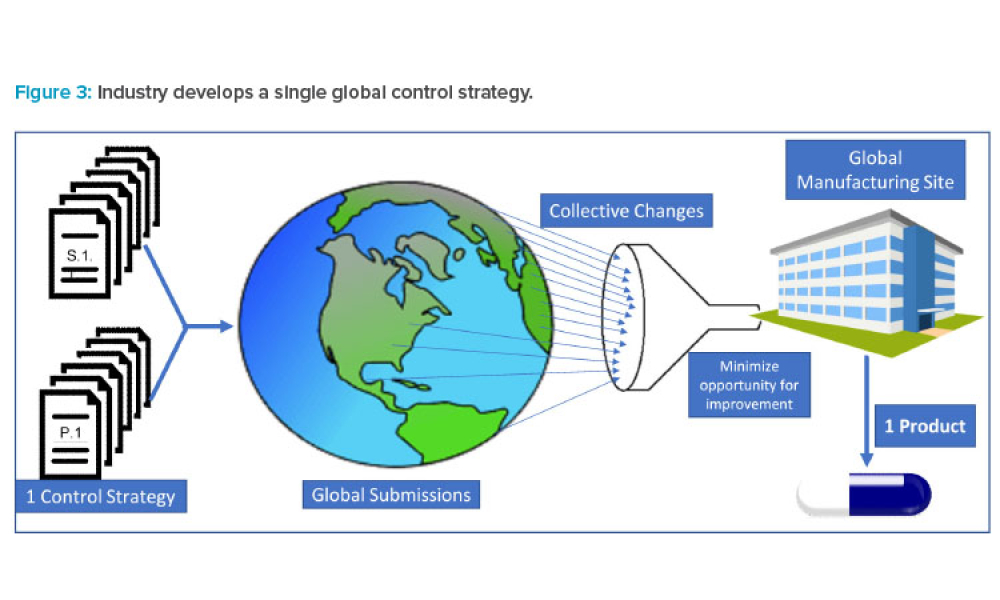
Regulatory Newsletter
This quarterly newsletter is dedicated to news about ISPE’s regulatory and quality activities. ISPE is committed to fostering communications and interactions to advance common interests among the pharmaceutical industry and regulatory agencies. ISPE provides a neutral environment where our individual Members and experts belonging to Health Authorities can engage in open dialogue on issues that will ultimately benefit patients around the world.