Winners in this category exemplify application of novel approaches, standards, and practices which result in efficient processing, resourceful utilities, and business advantage by increasing patient access and preventing drug shortages through in-country-for country manufacturing; outbreak, epidemic, or emerging health crisis response via rapid deployment and fast-track drug production; and...
Do you have your finger on the pulse of regulatory concerns in the Asia-Pacific Region? Would you like to discuss strategies to meet regional challenges? Are you interested in regulatory harmonization? If so, we’d like to re-introduce ISPE’s Regulatory Quality Harmonization Committee (RQHC)’s Asia-Pacific Regional Focus Group (A-P RFG).
Website: https://veqtorsolutions.com/what-we-do/
Our Technical Services provide:
-
Fast access to the right consultants with cutting edge knowledge, skills, and the expertise and attention to detail that you need. Our consultants serve clients throughout the United States and abroad.
At the 2022 ISPE Europe Annual Meeting held in Madrid on 25-27 April 2022, Evdokia Korakianiti, Head of Quality and Safety, European Medicines Agency (EMA), gave a keynote presentation of EMA’s approach to...
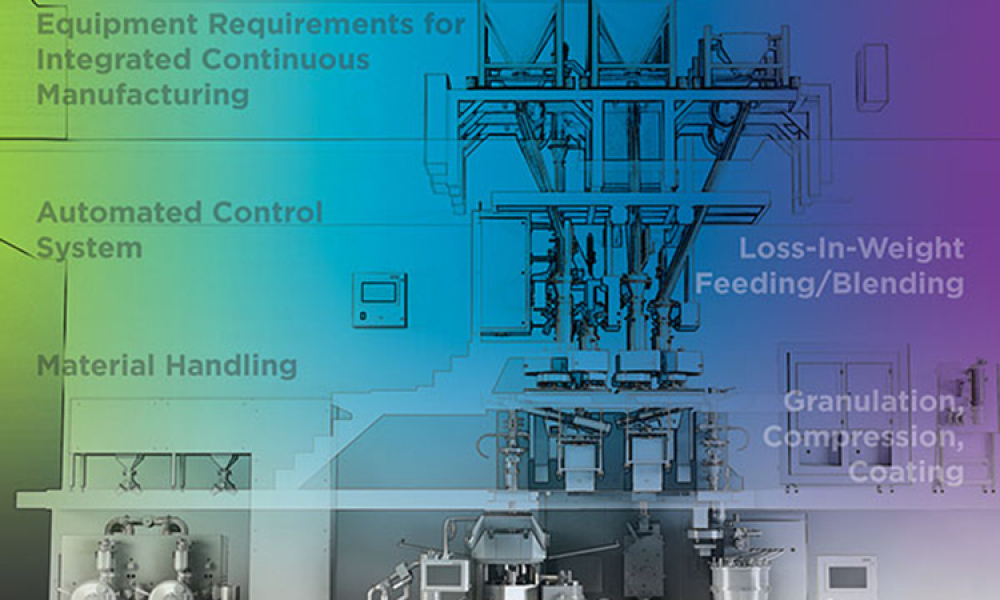
As the benefits of continuous manufacturing became more apparent in the pharmaceutical industry, companies looked for ways to incorporate the technology into their manufacturing processes. In 2017 The ISPE Oral Solid Dosage (OSD) Community of Practice (CoP) formed a working team to advance the use of continuous manufacturing in the pharmaceutical industry and to increase the long-term...
Are you considering joining ISPE? Or maybe you’ve recently joined the society or are a longtime member looking to better understand all the benefits available to you. If so, you’ve come to the right place!
ATMPs are based on genes, cells or tissues delivered to patients to provide a therapeutic benefit based on a specific target of interest. Often referred to as ‘Personalised Medicine’. A sector of healthcare that is rapidly evolving and expanding with some unique challenges such as microbial contamination and product variability. Traditional manufacturing processes are for synthetically derived...
GAMP® 5 Second Edition is here! Since its publication, GAMP® 5 has been far and away the leading international guidance on GxP computerized systems validation and compliance, and it was time to update our guidance to...
That question was at the heart of the of ISPE’s “Expert Xchange: Regulatory Summit on ICH Q9 Revision” held 9 June 2022. Seventy-one participants from 14 countries discussed ICH Q9(R1) and received valuable insight from several members of the Expert Working Group (EWG) assembled in 2020 to lead the ICH Q9 revision process. Discussion centered on the current state of application of quality risk...