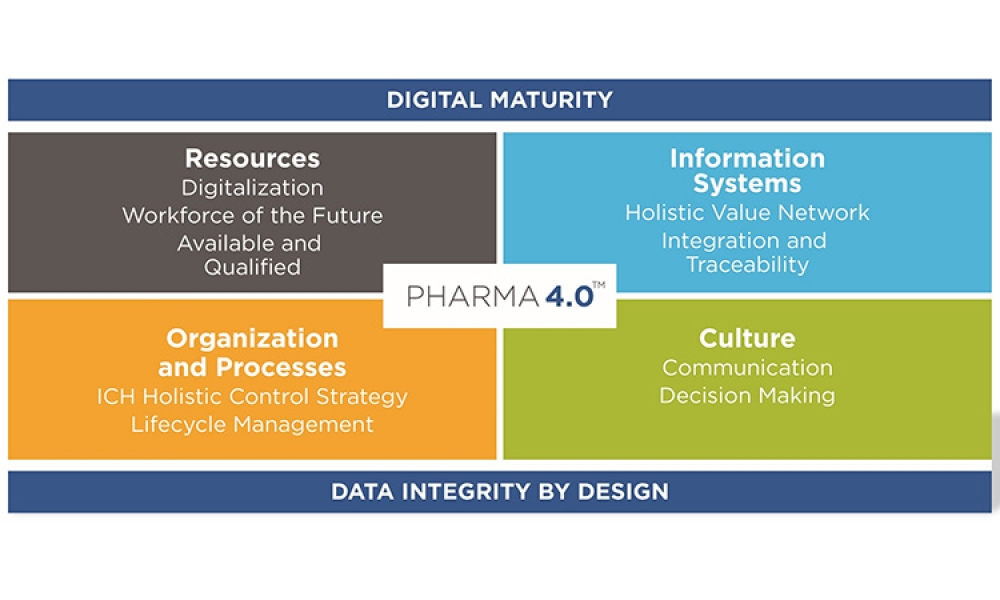
Innovation is an integral part of corporate strategy. Initiatives to facilitate innovation are continually developed and pursued. The 2022 ISPE Pharma 4.0™ Emerging Leader (EL) Hackathon was designed based on innovator needs and provided a hands-on blueprint manufacturing exercise. Over a period of two days and facilitated by 40 subject matter experts, coaches, and jury members, 50...
November / December 2023
In August, ISPE unveiled a new Guidance Documents Portal that provides a significantly improved online user experience for ISPE’s full library of Guidance Documents.
Since 2005, ISPE’s Facility of the Year Awards (FOYA) have recognized state-of-the-art projects utilizing new, innovative technologies to improve the quality of products, reduce the cost of producing high-quality...
The field of advanced therapeutic medicinal products (ATMPs) has witnessed remarkable advancements in recent years. The Emerging Leaders (EL) community from the Belgium and Germany/Austria/Switzerland (D/A/CH) affiliates collaborated to develop an inclusive series of online seminars on ATMPs. This article gives highlights of the speakers, topics, and knowledge shared in ATMP online seminars...
Process capability is a fundamental concept for manufacturers. Pharmaceutical, biopharmaceutical, and medical device manufacturers leverage capability analysis along with other statistical quality control (SQC) techniques to enable timely supply of quality medicine to patients.
The 2023 ISPE Biotechnology Conference opened on 26 June with a series of six keynote presentations on biotechnology and the development and manufacturing of advanced therapies. Tom Hartman, President and CEO of ISPE, introduced each of the keynote speakers.
Erich H. Bozenhardt is the Associate Director of Process Engineering in Regenerative Medicine Operations at United Therapeutics. Erich is an experienced bioprocess subject matter expert and internationally recognized authority in the areas of cell and gene therapy and bioprocessing. He has published more than 30 technical papers and is a frequent presenter at conferences.
Michelangelo Canzoneri, PhD, is a seasoned leader in the digital transformation space within the healthcare and life science industry currently serving as the Global Head of Group Smart Manufacturing at Merck KGaA, Darmstadt, Germany. In this pivotal role, he functions as the primary business interface across the life science, healthcare, and electronics sectors, steering the incubation,...
Live biotherapeutic products (LBPs) have the potential to treat a wide range of ailments. However, these living microorganisms are difficult to produce due to evolving government regulations and limited GMP manufacturing experience. New facility designs and more specific process guidance could help overcome these challenges. This article explores the nuances of facility design and regulatory...
Cell and gene therapy (C>) products comprise a rapidly growing field of innovative medicines that hold the promise to treat and, in some cases, cure diseases that are otherwise untreatable. In this article, we provide points to consider when evaluating the comparability of C> when changes are made in their manufacturing processes.