May / June 2020
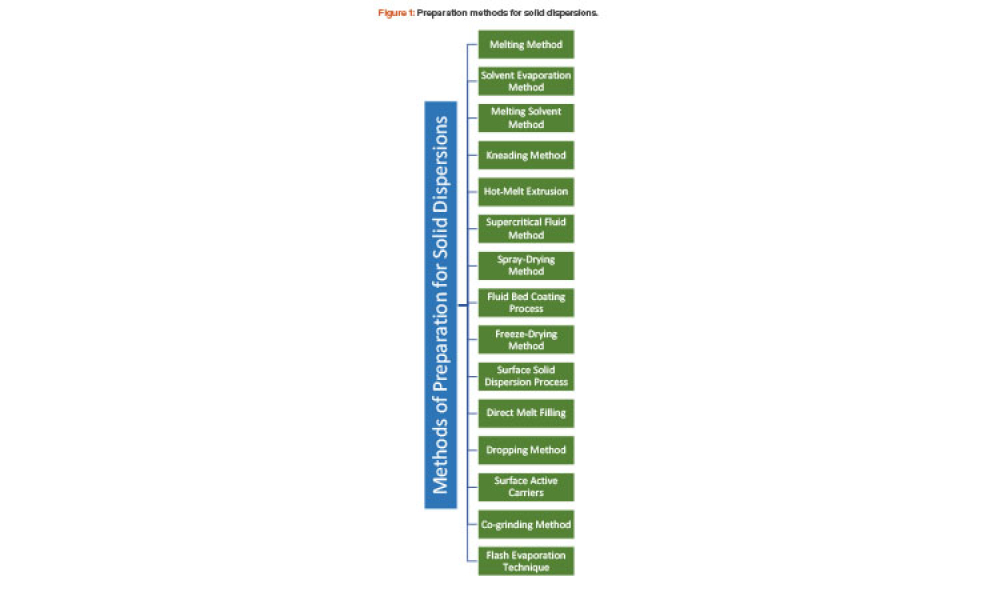
The solubility behavior of drugs remains one of the most challenging aspects of formulation development and is a key determinant of a drug’s bioavailability. This article describes research aimed to improve solubility of a poorly water-soluble drug (ibuprofen) by preparing a porous solid dispersion using a flash evaporation technique.
Over the years, the roles and responsibilities of engineering and quality/validation personnel for commissioning and qualification (C&Q) activities have evolved. Now more than ever, commissioning and qualification approaches based on quality risk management (QRM) principles rely heavily on engineering and the application of Good Engineering Practice (GEP) to provide documentation for the...
Pharmaceutical Engineering® has launched another new section on the Pharmaceutical Engineering Online site: Online Exclusives. Pharmaceutical Engineering Online Exclusives are articles published exclusively on the Pharmaceutical Engineering website. This new feature expands the...
Brazil’s regulatory authority is working hard to make the nation a larger player in the global pharmaceutical market, and these efforts appear to be working: the life sciences industry has expanded in recent years, and market projections are positive. These ongoing developments represent opportunities for the ISPE Brazil Affiliate as it undergoes its own transformation.
As the old saying goes, “Time is money.” In today’s industrialized world, this adage is profoundly true. Manufacturers can no longer afford to overlook operational excellence. A new production philosophy called “Lean manufacturing” has been developed to save as much time as possible during manufacturing processes. In some industries, such as the automotive sector, Lean has almost been...
Pam Cheng is Executive Vice President, Global Operations & Information Technology, at AstraZeneca, a United Kingdom–headquartered pharmaceutical company with more than 60,000 employees. In this role, she combines her expertise as an engineer with business savvy and seeks opportunities to lead her company and her industry forward in innovative ways.
This article introduces the concept of robotic process automation (RPA) and discusses how the technology may be used within a GAMP® framework to support both non-GxP and GxP processes.
At the 2019 ISPE Global Pharmaceutical Regulatory Summit, regulators updated attendees on approaches to industry innovations and the ongoing work on harmonization and reliance around the...
Virtually every ISPE member has at least one story to tell about how health authority inspections or the review and approval of regulatory applications have affected their efforts to supply critically needed medications to patients globally. Although these stories may emphasize the considerable challenges that ISPE members face, they also frequently identify great opportunities for innovation...