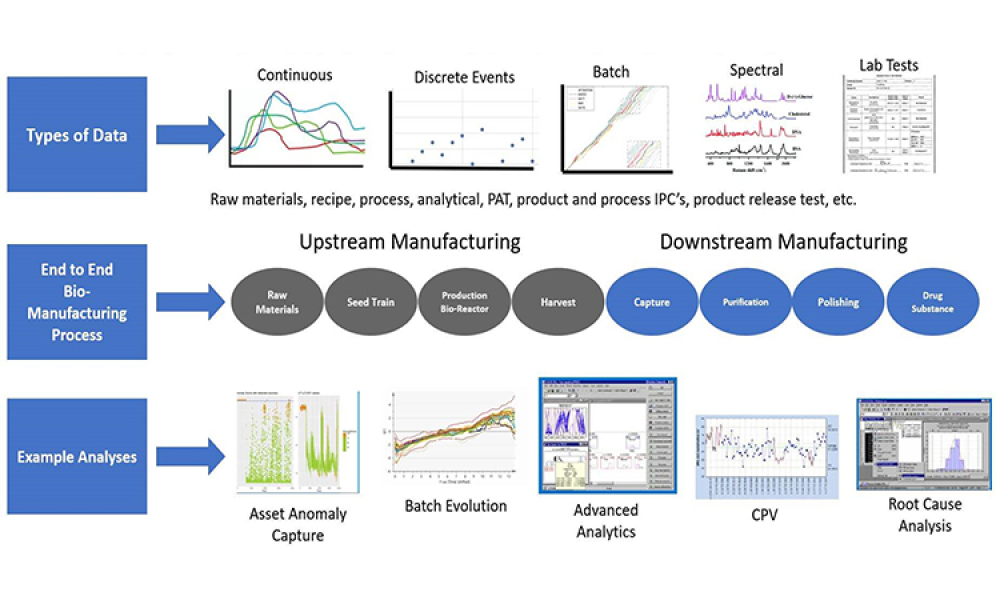
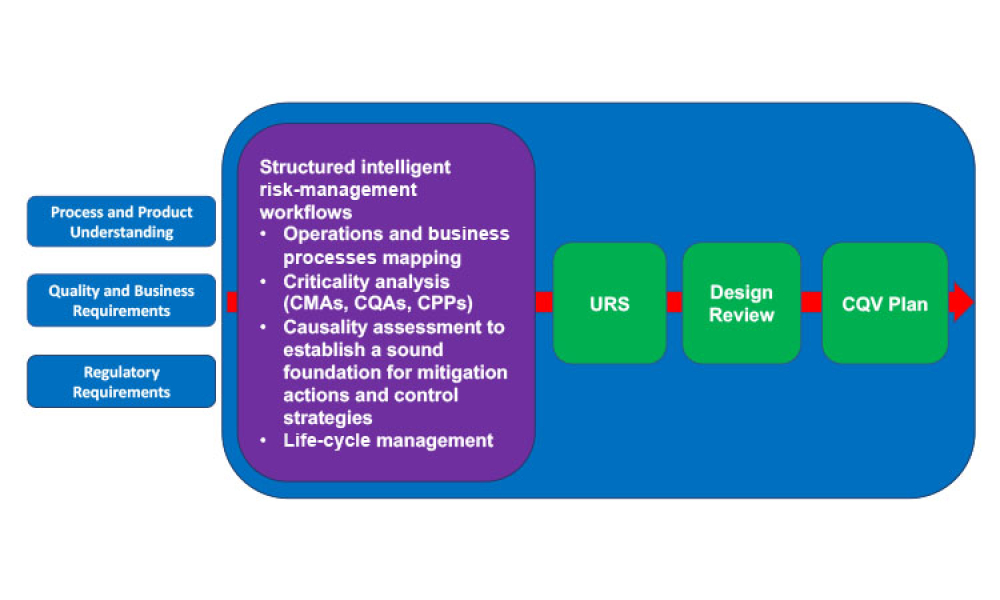
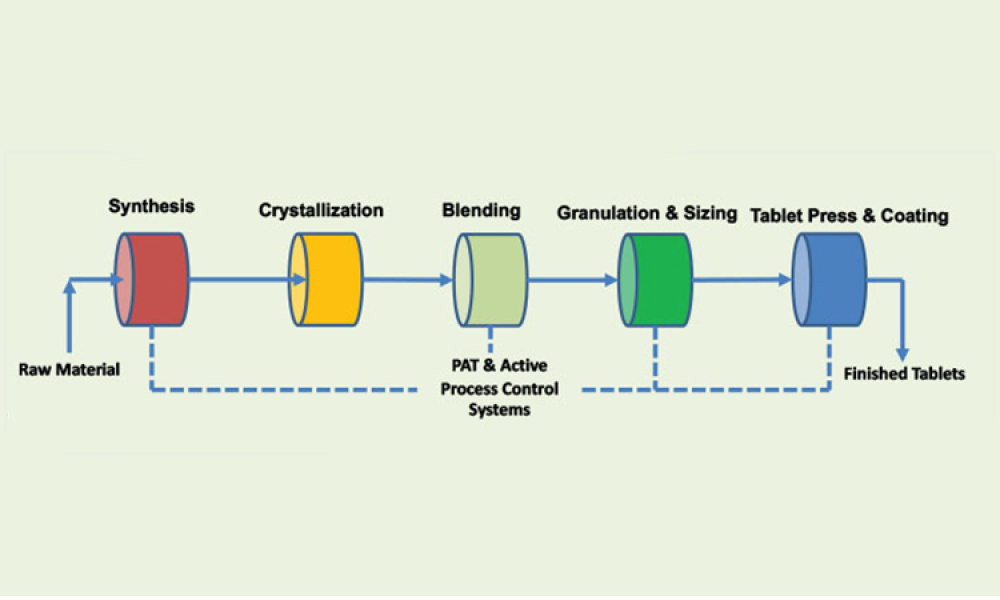
A growing segment of the advanced therapy medicinal product (ATMP) landscape, which includes gene therapies and cell-based treatments, relies heavily on viral vectors for efficient gene delivery. The increasing demand for these therapies requires a robust, scalable, and cost-effective manufacturing solution.
Robert Dream

Nivagen Pharmaceuticals, Inc.
Vice President, Quality
Robert Dream is a distinguished leader in the biopharmaceutical industry, bringing over 34 years of comprehensive experience that spans the full product lifecycle—from early-stage process development to large-scale commercial manufacturing. His deep expertise encompasses a diverse array of biopharmaceutical products, including vaccines, recombinant proteins, monoclonal antibodies (mAbs), and advanced therapy medicinal products (ATMPs), such as cell and gene therapies (C>s).
Throughout his career, Robert has been instrumental in driving success across critical areas including manufacturing operations, regulatory compliance, facility design and qualification, process development and validation, and strategic CAPEX/OPEX planning. He has played a pivotal role in supporting global expansion efforts for numerous organizations, offering expert guidance in CMC strategies, audits, and regulatory submissions, including BLA and MAA filings.
Robert has led major capital and operational projects, expertly managing contracts, procurement, and program delivery across international markets. His insights have been published in prominent industry textbooks and peer-reviewed technical journals. A recognized thought leader, he is a frequent speaker at industry seminars and technical conferences, and has served as a lecturer at universities and training institutions.
An active member of the Parenteral Drug Association (PDA) and the International Society for Pharmaceutical Engineering (ISPE) since 1990, Robert continues to shape the future of biopharmaceutical manufacturing through his leadership, technical contributions, and commitment to excellence.
Throughout his career, Robert has been instrumental in driving success across critical areas including manufacturing operations, regulatory compliance, facility design and qualification, process development and validation, and strategic CAPEX/OPEX planning. He has played a pivotal role in supporting global expansion efforts for numerous organizations, offering expert guidance in CMC strategies, audits, and regulatory submissions, including BLA and MAA filings.
Robert has led major capital and operational projects, expertly managing contracts, procurement, and program delivery across international markets. His insights have been published in prominent industry textbooks and peer-reviewed technical journals. A recognized thought leader, he is a frequent speaker at industry seminars and technical conferences, and has served as a lecturer at universities and training institutions.
An active member of the Parenteral Drug Association (PDA) and the International Society for Pharmaceutical Engineering (ISPE) since 1990, Robert continues to shape the future of biopharmaceutical manufacturing through his leadership, technical contributions, and commitment to excellence.