Pharmaceutical and biotechnology companies employ platform analytical procedures in the development stages of their synthetic and biological drug products and are beginning to leverage them for commercial products. This shift is supported by the acceptance of platform procedures in the recently adopted ICH Q2(R2) and ICH Q14. Six case studies are shared in this article to highlight how...
Nina Cauchon, PhD

Nina is active in several external organizations which provide a strong network and knowledge base, including being a speaker/committee member for ISPE, CASSS, PQRI, AAPS, IQ, and DIA. She is a member of the ISPE International Board of Directors, the PhRMA Global Quality and Manufacturing group, and the ICH Q2(R2)/Q14 Expert Working Group, and is currently the ISPE PQLI co-chair. She has a PhD from Purdue University School of Pharmacy and has over 25 years of industry experience.
Related Articles
The pharmaceutical industry faces considerable challenges throughout the development, manufacturing, and supply of medicines, largely due to the intricate and divergent global regulatory landscape. The adoption of structured data standards and utilization of cloud-based platforms offer immense potential to overcome these challenges by facilitating faster and more efficient global...
The biopharmaceutical industry must develop and implement innovative ways of working to be effective and efficient in the current healthcare ecosystem, in which high-quality medicines, adaptability, and assurance of supply are of critical importance. There are regulatory strategies and technologies emerging to address these challenges, but further progress must be made to fully harness the...
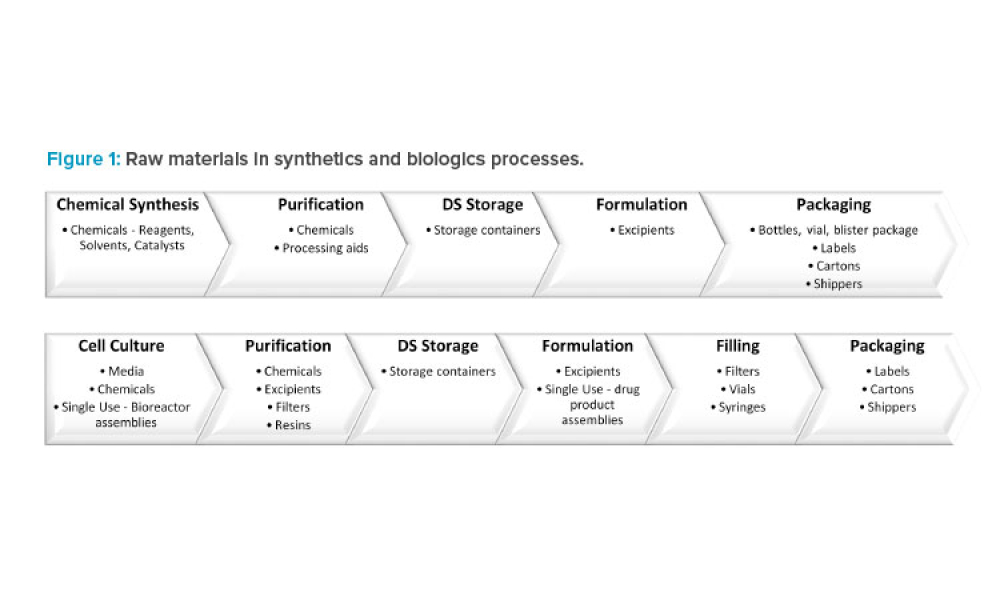
Pandemic-related supply chain shortages have placed constraints on the supply of essential filters and chromatography resins. An agile regulatory pathway to implement alternative filters and resins into manufacturing is necessary to ensure the continued supply of approved biologics. To allow this in the US and potentially globally, the regulatory strategy proposed in this article is to provide...
A reliable supply of raw materials is critical to maintain a robust supply chain to serve patients globally. With shortages, regulatory complexity is compounded due to differences in submission and data requirements from various regulatory agencies. Therefore, there is an increasing need to implement a harmonized regulatory infrastructure that is both flexible and predictable to provide more...
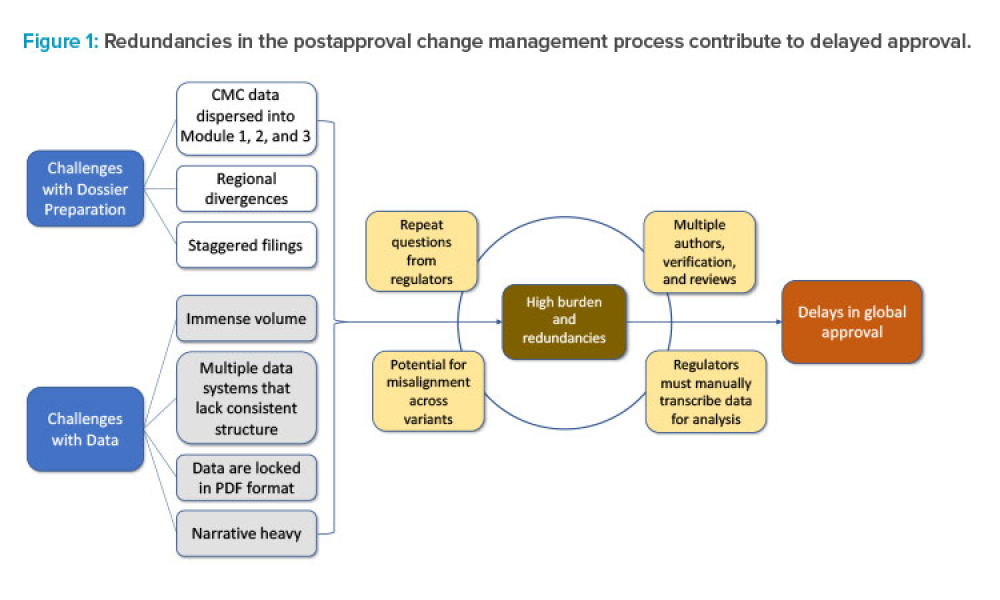
Postapproval change management of pharmaceuticals is an essential part of life-cycle management but is associated with regulatory challenges. Incorporating concepts and tools from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q12 guideline, combined with structured content and data management (SCDM) and a cloud-based data exchange...