We are The Culture Club, a self-formed cross-CoP group of individuals who came together to discuss challenges encountered as we transition quality and validation into the digital world and identify ways to influence cultural changes needed within our industry to better enable innovation. As we are not all from the same CoP, we first needed to establish a way to connect and collaborate with all...
On 17 December 2022, more than 100 people from all over the world gathered online for the 2022 China Pharmaceutical Supply Chain Summit hosted by Shanghai ISPE Pharmaceutical Information Company (SPIC). With multiple geopolitical and pandemic related challenges in recent years, having a robust supply chain has increasingly become a strategic advantage for the pharmaceutical industry. The...
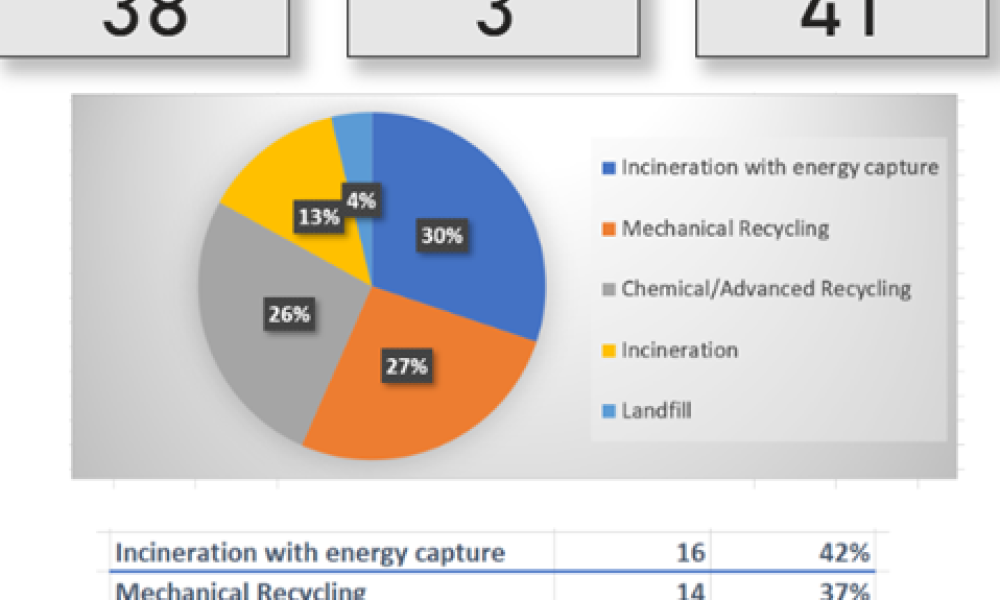
In the iSpeak Blog posting on 14 March 2023, the first part of this blog series introduced the results of a survey that was conducted of users of single-use assemblies. The focus...
Members of the paperless validation subcommittee created this blog post to discuss and recommend “true copy verification practices” for the use of Paperless validation systems to satisfy the current guidance for data integrity, and to discuss how to fully eliminate paper from various validation processes without impacting compliance to these current regulations.
The Biopharmaceutical industry continues to grow and deliver life-changing medicines to patients as evidenced by the number of drug approvals by the FDA year after year. In 2022 alone CDER approved 37 novel drugs, either as new molecular entities (NMEs) under New Drug Applications (NDAs) or as new therapeutic biological products under Biologics License Applications (BLAs). This has all been...