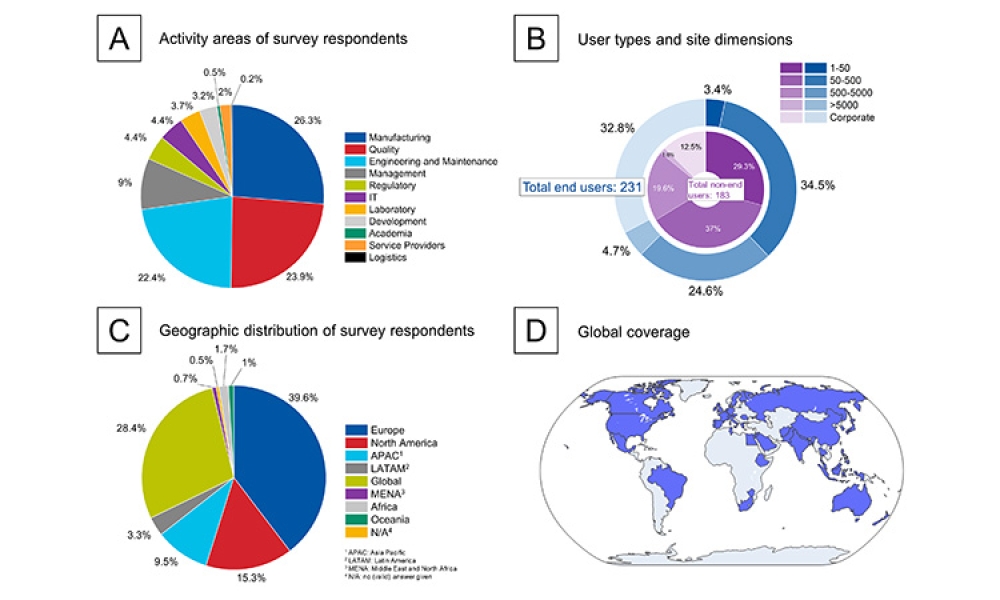
This article summarizes the key findings from the 7th Pharma 4.0™ Survey, conducted in 2023. It explores the demographics of the survey respondents, maturity levels of Pharma 4.0™ adoption, enabling technologies being leveraged, anticipated benefits, and challenges encountered.
September / October 2024
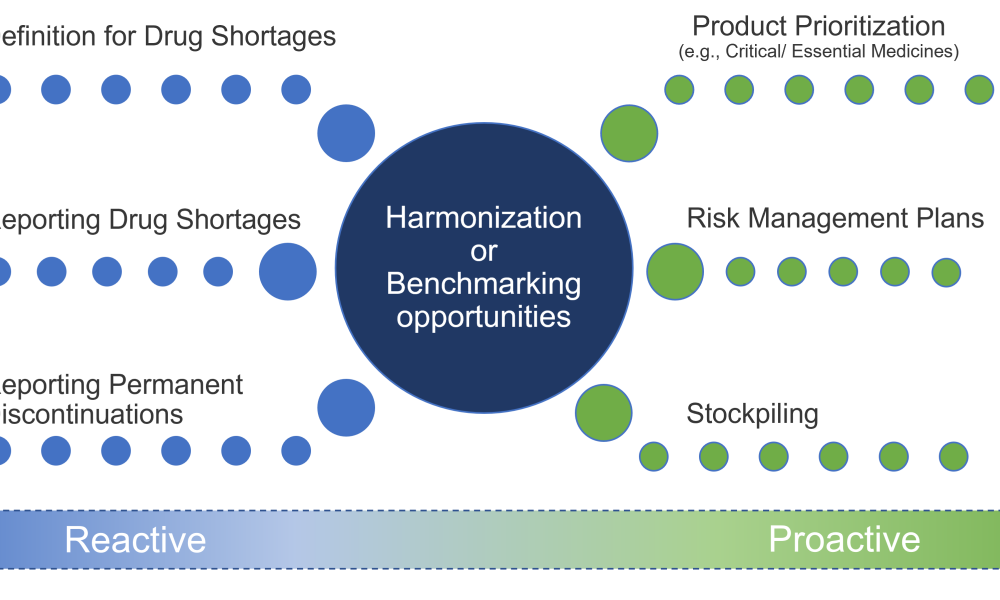
The regulation of drug shortage prevention has rapidly changed over the past several years due to large-scale, highly visible events, such as the COVID-19 pandemic, hurricanes, and geopolitical issues. Efforts to effectively address the complex and multifaceted issues contributing to drug shortages require close technical collaboration and clear communication between the pharmaceutical...
The pharmaceutical landscape is rapidly evolving, and cell and gene therapies (C>) are at the forefront of this transformation. These therapies are revolutionizing how we approach patient care, particularly in the realm of personalized medicine. However, this innovation has also introduced challenges, especially when establishing new manufacturing facilities.
Drug delivery devices have become an essential component for many modern medical therapies, and it’s vital that they function as intended. However, the reality of marketed products shows that this is not always achieved because drug-device combination products are becoming increasingly complex, with an increasing number of potential failure modes. Significant challenges for engineers include...
Computer software assurance (CSA) has been discussed widely in industry over the past five years. While the principles are well understood and welcomed, until now some of the practical detail on how exactly to implement CSA into an organization has been missing.
Cell and gene therapy (C>) products address various diseases at the cellular or genetic level, offer innovative treatment approaches, and represent a significant advancement in the field of medicine. However, developers of C> products face unique challenges due to their complexity, such as establishing assays that show a clear link between potency, mechanism of action (MoA), and...
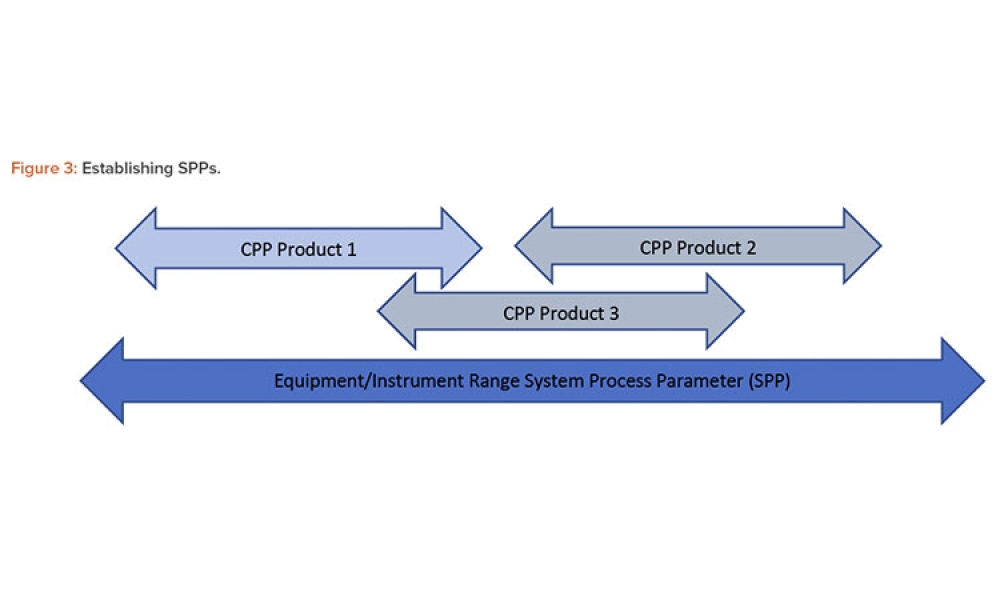
In 2011, the US Food and Drug Administration (FDA) introduced the revised “Guidance for Industry: Process Validation: General Principles and Practices.”
In 2023, ISPE launched an expansive and significant initiative, Enabling Global Pharmaceutical Innovation: Delivering for Patients, to address the barriers to technological innovation in the pharmaceutical industry. The first activity of the initiative was to conduct a three-part survey of ISPE members to understand the circumstances and confirm the sources that create barriers to...
After completing her doctoral degree at Oklahoma State University, Sarah Pope Miksinski received a fellowship at the National Institutes of Health. While working there, she realized that research was not the best career path for her.
Anil Mathai first heard about ISPE 30 years ago. “I attended Drexel University, where you are required to complete three cooperative education jobs. One of mine was for Rhône-Poulenc Rorer, Inc. in Collegeville, Pennsylvania. While I was there, I learned about validation and decided that I wanted to be in pharmaceuticals as a chemical engineer.”