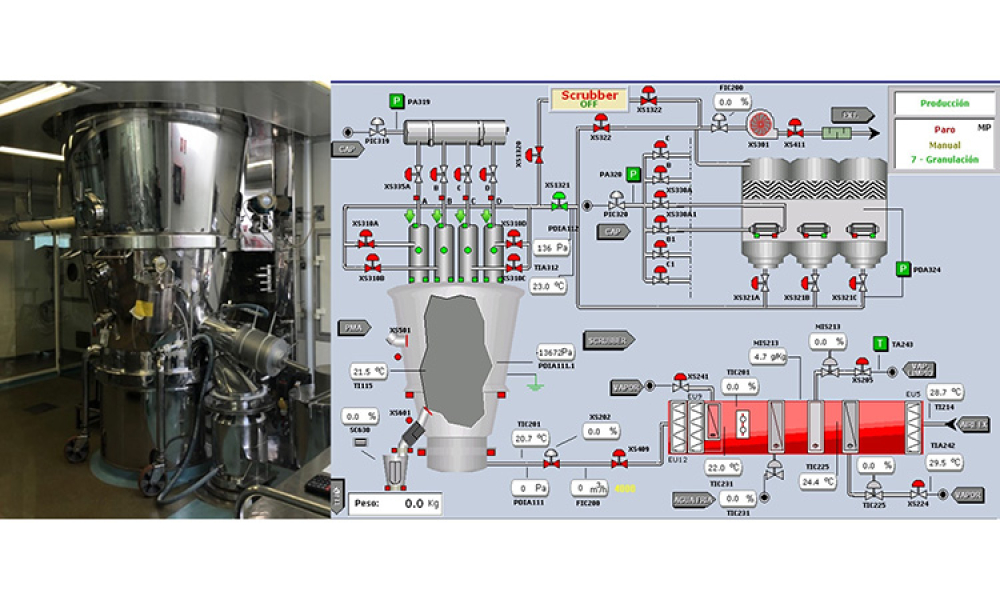
This article shows how non-supervised artificial intelligence (AI) methods can strongly support common processes in the pharmaceutical industry such as wet granulation drying. The techniques used demonstrate how continuous process optimization can be enabled and the process control strategy is permanently updated while keeping product quality under strong assurance and also increasing energy...
November / December 2022
An interview with Shin Kawamata of Japan’s Foundation for Biomedical Research and Innovation (FBRI) highlights exciting work to move cell therapy toward reliable and scalable commercialization.
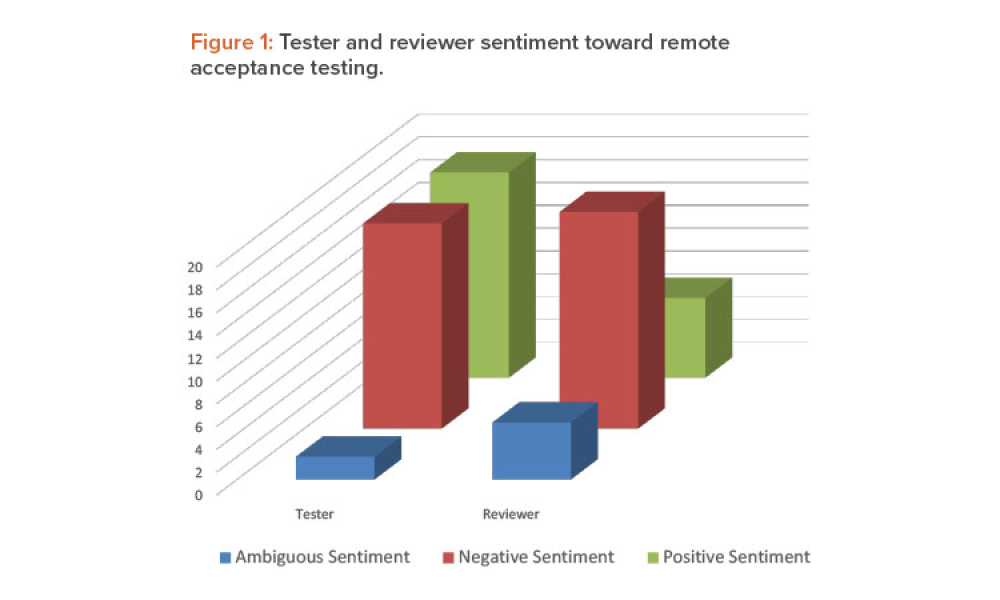
Before the COVID-19 pandemic, it was unthinkable for a system integrator to suggest remotely conducting an acceptance test for an automation project. This article shows how automation engineers and client validation personnel were successful in navigating COVID-19 restrictions and overcoming previously held preconceptions about remote testing to meet end-user and regulatory requirements....
This artificial intelligence (AI) retrofit project was a unique approach to implementing AI technology in a pharmaceutical environment within three months. This project tackles a commonly known industry challenge by integrating AI into an existing automatic visual inspection (AVI) machine. The proof of value allowed us to benchmark added value through AI compared with state-of-the-art...
Combination Products is one of ISPE’s newest Communities of Practice (CoPs). It started as a Special Interest Group to help people in the industry collaborate and learn from each other.
When the ISPE Baseline Guide Vol. 5, Commissioning & Qualification, 2 ed. was published in 2019, most of the attention was focused on the incorporation of quality risk...
For nearly a century, production of water for injection (WFI) was universally accepted to be distillation-based. As emphasis on costs and environmental concerns has grown, pharmacopeias around the world have focused on the quality attributes of WFI to allow for consideration of other production technologies.
More than 400 attendees learned about the latest developments in biopharmaceuticals, cell and gene therapy, and ATMPs at the 2022 ISPE Biotechnology Conference, held 28–30 June in Boston, Massachusetts.
Resounding clinical successes and maturation of extensive therapeutic pipelines have catapulted oligonucleotides from a fringe modality to therapeutic relevance in just a few short years. Oligonucleotides are a cornerstone of a burgeoning class of drugs classified as nucleic acid therapeutics. These therapies interact with DNA and RNA targets rather than traditional protein therapeutic...
Cell and gene therapies (C>) have unique needs in manufacturing suites that differ from those for classic product biopharmaceuticals. Facilities must be created with flexibility in mind, able to run multiple products and production types to remain viable.