Powder for oral suspension (PfOS) bioavailability is mostly on the basis of drug absorption from the gastrointestinal tract. PfOS formulation pH, viscosity, vehicle buffer capacity, drug particle size distribution, density, and viscosity are often critical for absorption. Therefore, careful design and selection of excipients—including suspending agents—are necessary during PfOS formulation...
July / August 2021
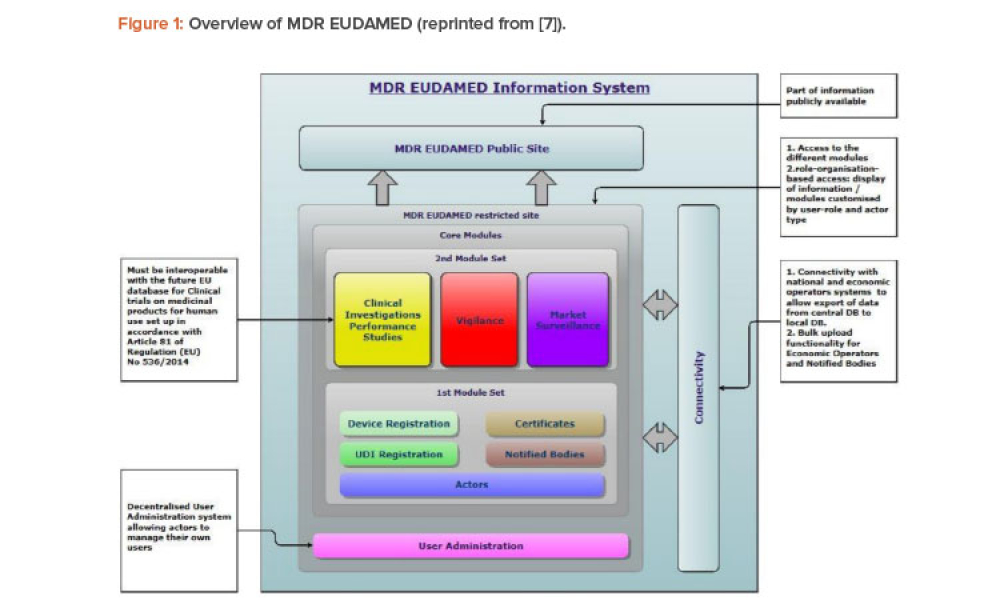
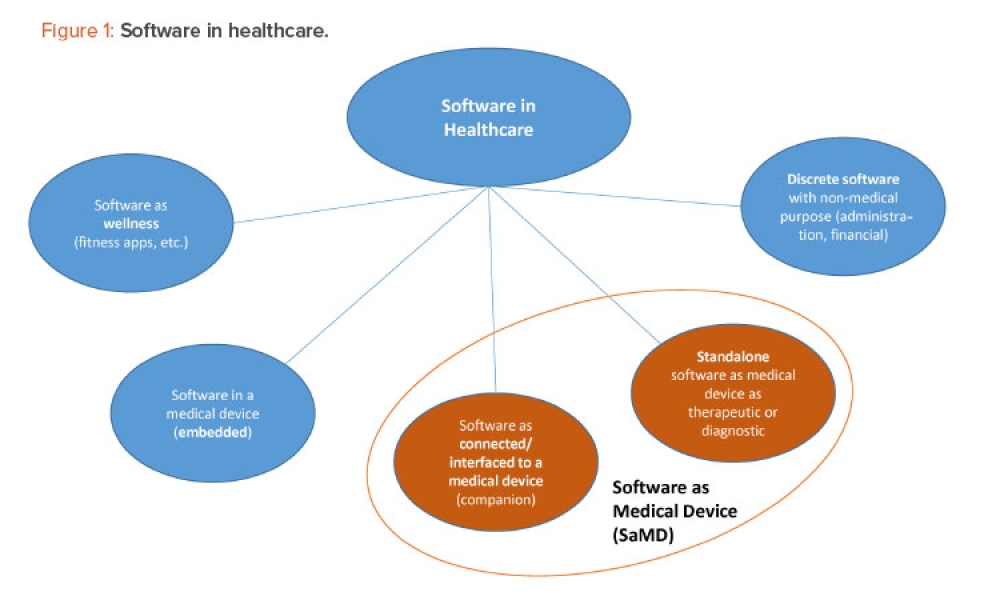
Since 2019, the ISPE France Affiliate’s Unique Device Identification (UDI) Medical Device Work Group has been producing tools to help project stakeholders within the EU or overseas understand and comply with EU regulations of UDIs in medical devices. Some of those tools are highlighted in the article.
The pharmaceutical industry in Spain and Portugal is growing, and the ISPE Iberia Affiliate—which has merged the Spain and Portugal Affiliates to take advantage of synergies between the two Affiliates—is working to train and support this expanding market.
The ISPE Foundation Diversity Internship Program has received strong response from applicants and has launched a new partnership.
Optimism is in the air as we move through the summer months and the restrictions that have become second nature are slowly lifting. As I continue the second half of my year as the Emerging Leaders (EL) Chair and representative on...
Continuous manufacturing has attracted significant interest over the past decade for small molecules formulated as drug products. The case for adopting continuous manufacturing platforms for manufacturing biologics (i.e., large proteins or biologic products such as vaccines) would, in principle, be even more justified for both quality and business gains. This article briefly reviews continuous...
Organizations wanting to ensure the well-being and growth of their employees need to invest in robust mentorship and recognition programs. Mentoring has played a key role in my career development. In the 1990s and early 2000s, I worked in Novartis and engineering services companies where I was part of informal mentoring groups with some senior directors and trusted collaborators across the...
Remembering the “why” of our pharma industry is so important and, for me, also very personal. My sister Linda was 69 and her four grandchildren were the light of her life. In November 2017, she was diagnosed with appendix cancer (a rare cancer, closely related to colon cancer). After four months of nasty chemotherapy, Linda entered hospice care and died the next month. Previously, my mother...
Cell and gene therapies are complex. As more therapies come to market in the hope of bringing advanced treatments and cures to rare, orphan, and difficult-to-treat diseases, designing quality standards for these personalized medicines is equally as complex.