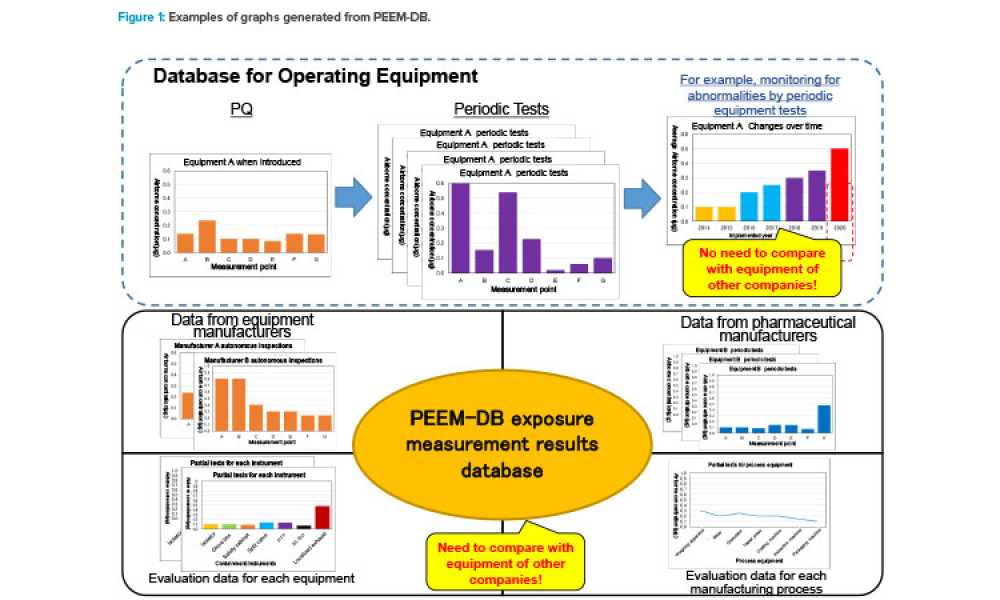
This article describes the Pharmaceutical Equipment Exposure Measurement Database (PEEM-DB), which was launched in July 2019 by the ISPE Japan Affiliate for its members. PEEM-DB is offered as a tool for rationally advancing optimal containment equipment settings by collecting exposure measurement results for...
January / February 2021
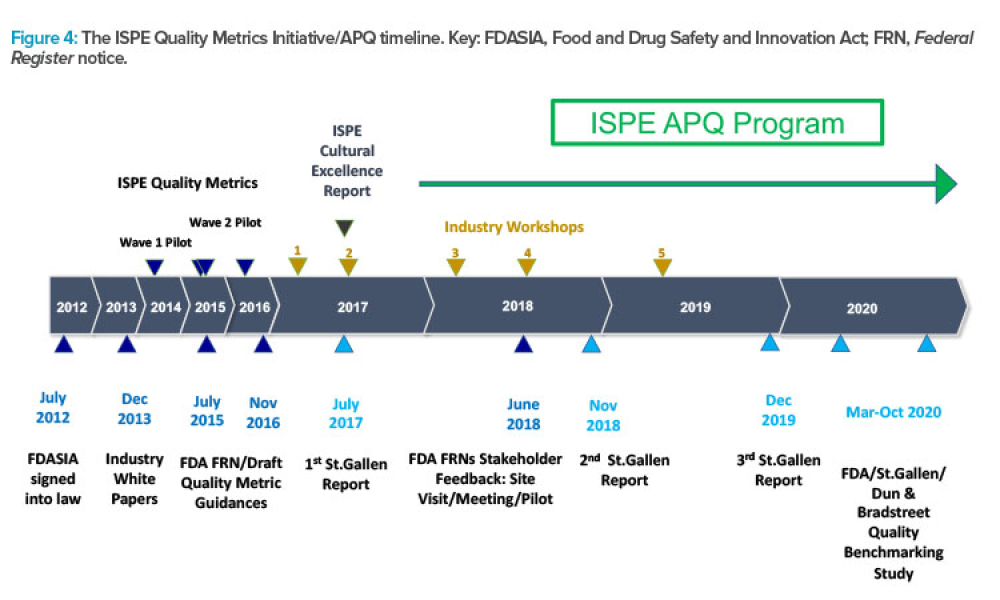
ISPE has announced the launch of its Advancing Pharmaceutical Quality (APQ) Program with the publication of the ISPE APQ Guide: Corrective Action and Preventive Action (CAPA)...
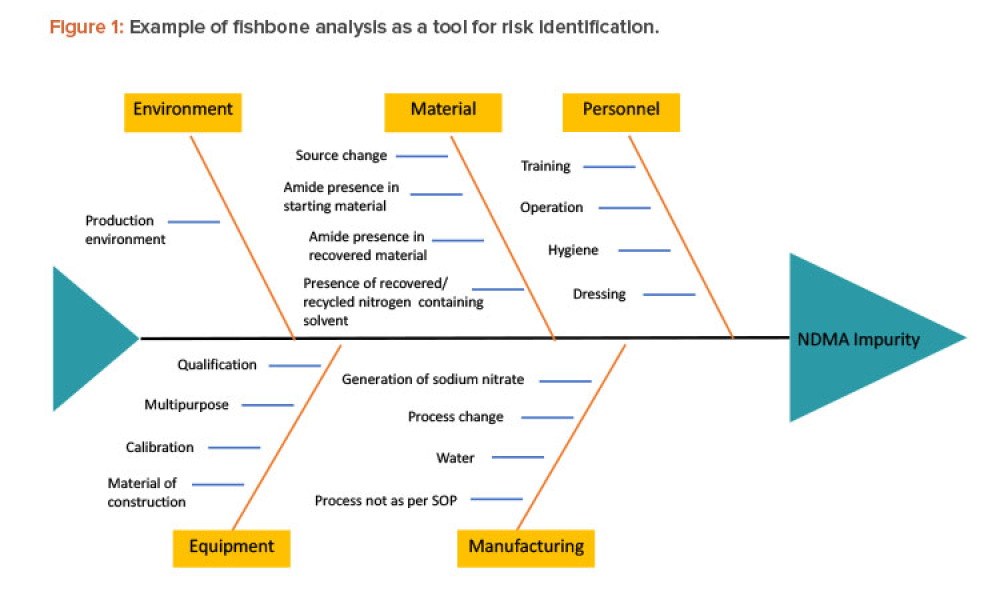
Recently, recalls of angiotensin receptor antagonists, particularly valsartan, and warning alerts about N-nitrosodimethylamine (NDMA) impurities in drug substances such as ranitidine and metformin have demonstrated the urgent need for manufacturers and regulators to control impurities throughout the product life cycle to ensure patient safety.
Drug developers know that the odds of anyone compound demonstrating safety and efficacy for a disease and its affected populations are low. How can drug developers improve these odds and increase the efficiency and effectiveness of drug development? One useful tool is model-informed drug development (MIDD), which uses computer models to inform the design of clinical trials or to run...
Personnel management is the most challenging variable in maintaining current Good Manufacturing Practice (cGMP) across the life cycle of drug manufacture, safety, and supply. A standard operating procedure (SOP) outlines agreed-upon instructions for personnel training and instructions for maintaining systems, machines, documents, and records in a qualified state to produce safe products. This...