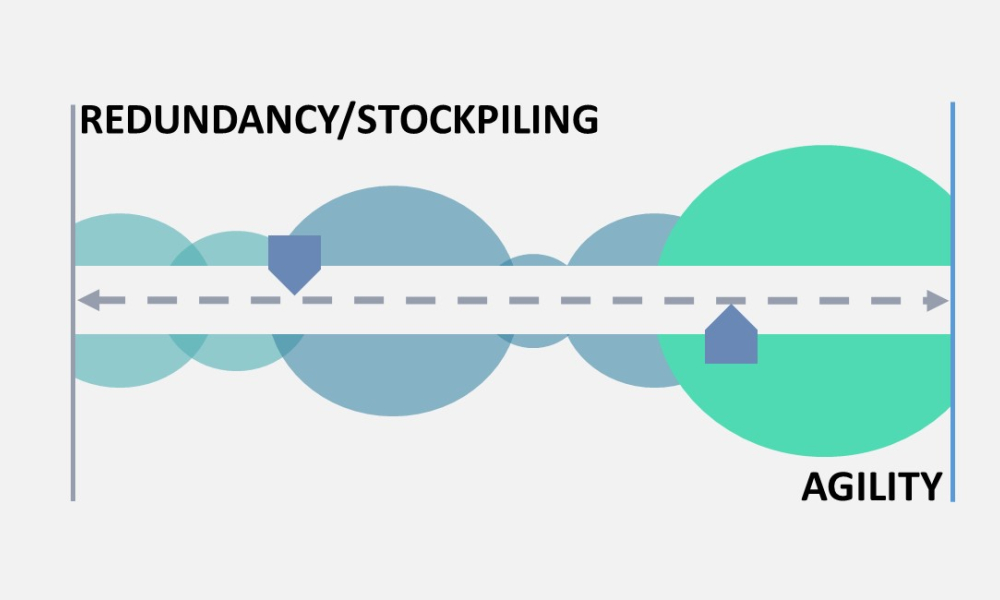
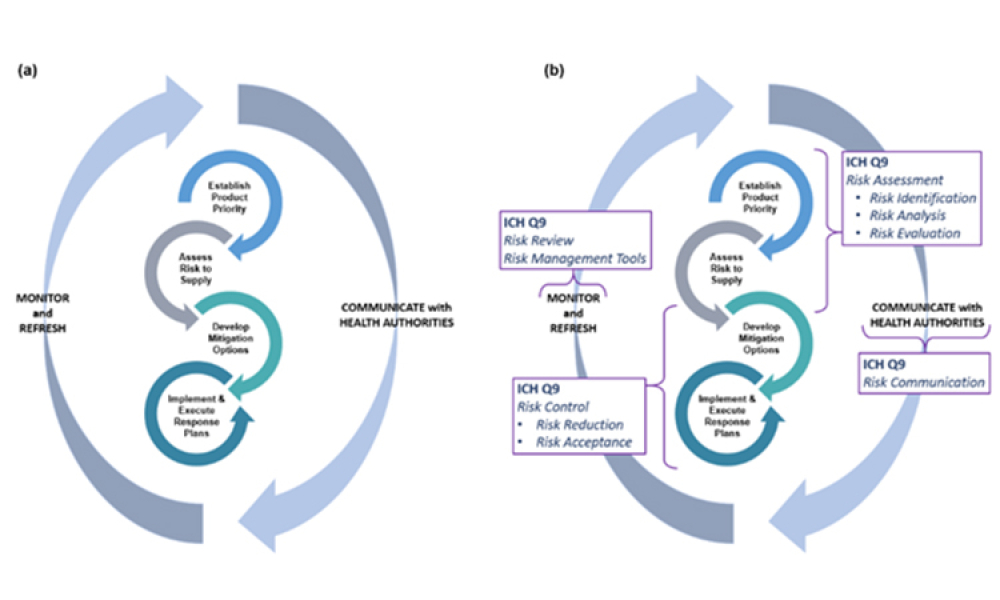
Supply resiliency for pharmaceutical manufacturing has been a topic of increasing interest to global stakeholders (Figure 1), resulting in widespread attention and many perspectives on how to improve drug shortages and associated vulnerabilities in the drug supply chain. At its core, supply resiliency is the ability for a manufacturer to have the agility, capacity, quality maturity, and risk...
Diane Hustead

Related Articles
Shortages of essential medicines around the world have been an ongoing concern for patients, caregivers, and regulators and have been exacerbated by the COVID-19 pandemic. Many regulators have instituted requirements for reporting potential or actual drug shortages.
In September 2021, a panel of regulators representing ANSM (France), ANVISA (Brazil), FDA (US), and the WHO participated in a webinar to discuss opportunities for drug shortage prevention. The discussion was facilitated by industry leaders while industry insights were obtained through polling of the global audience.
As noted in the ISPE Drug Shortages Prevention Plan,
As noted in the ISPE Drug Shortages Prevention Plan,