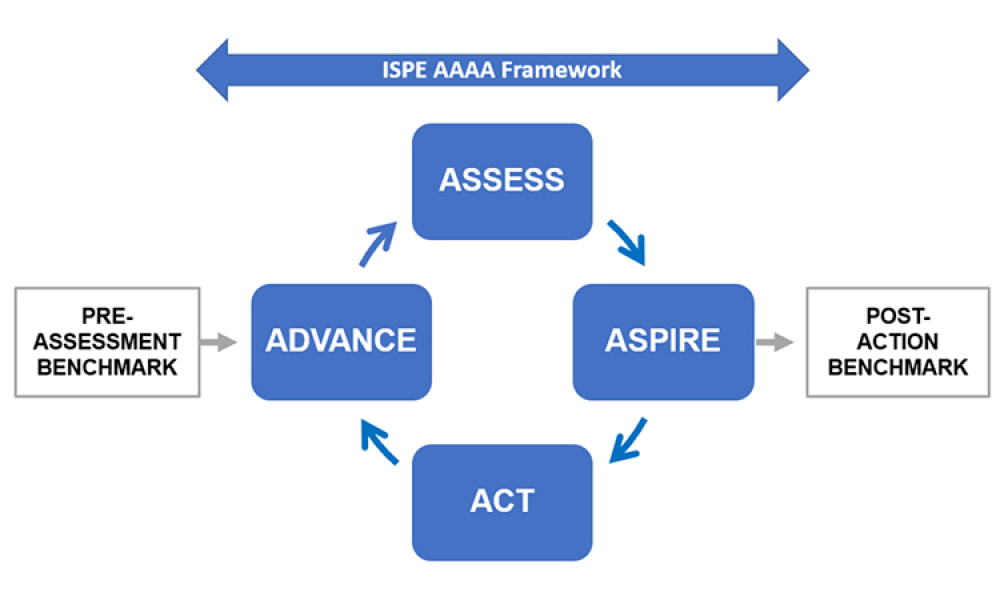
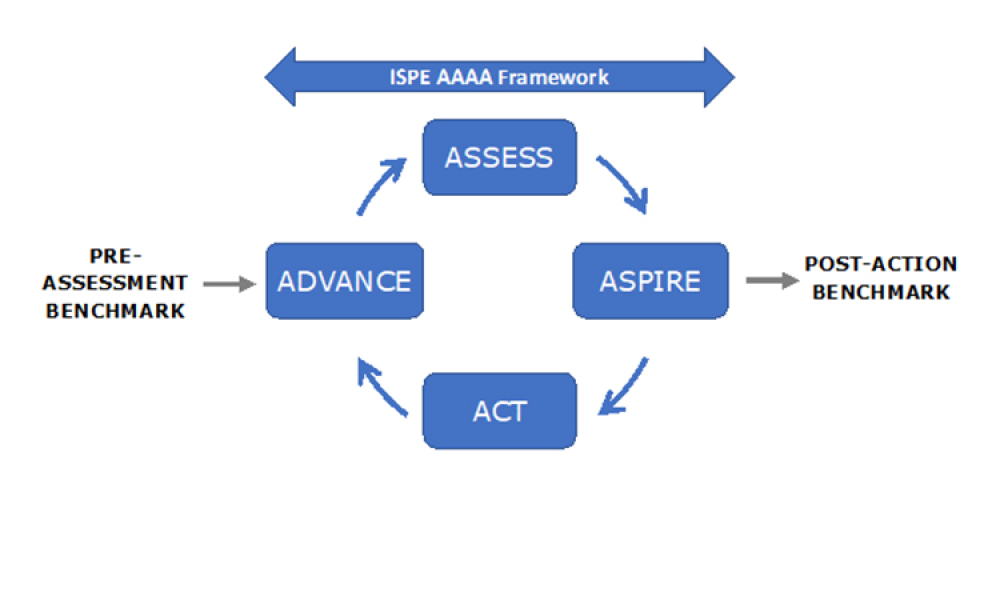
ISPE is a global industry leader in scientific, technical, and regulatory advancement throughout the entire pharmaceutical lifecycle. As a result, ISPE is in a strong position and available to assist the US government, its allies, and like-minded regulatory partners with implementation of recommendations emerging from a report,
Christopher Potter, PhD

Related Articles
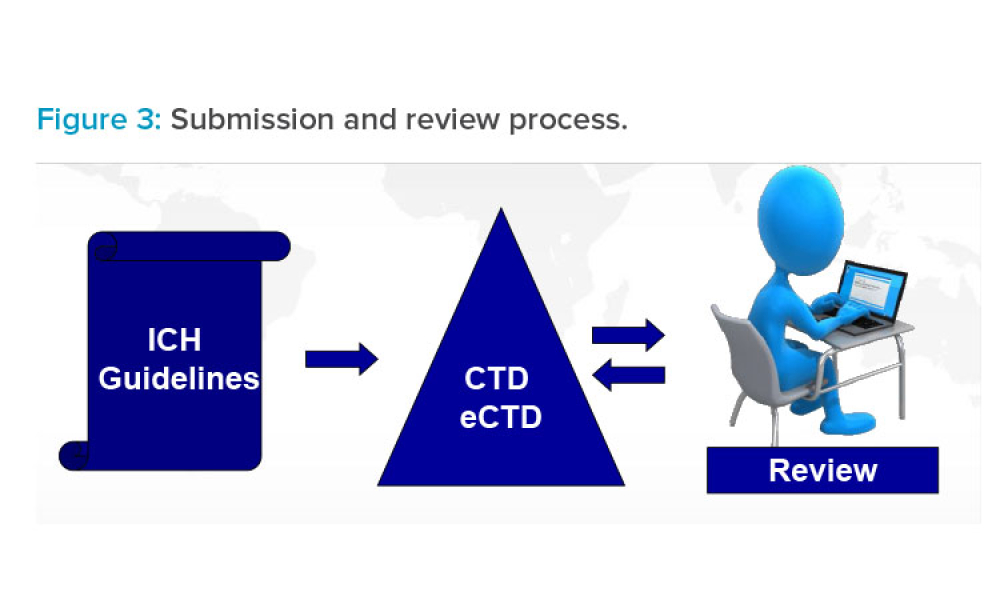
In its 30-year history, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has covered a wide range of topics to generate quality, safety, efficacy, and multidisciplinary harmonized guidelines. As science advances, issued guidelines are being updated and new guidelines are proposed in a pipeline of potential future activity. This...
The ISPE Global Pharmaceutical Regulatory Summit, held virtually on 28 April 2021, brought together 11 regulators from different parts of the world to discuss how their...
ISPE’s second virtual Global Pharmaceutical Regulatory Summit, held on 16 June 2021, brought together regulators from European Medicines Agency (EMA), Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration, and Center for Drug Regulation and Research, FDA Philippines to share their insights on application of quality risk management. They discussed the positive...
ISPE continues to work with a group of industry associations to assist the European Union (EU) and the European Medicines Agency (EMA) with implementation of revision of Annex 1, Manufacture of Sterile Products....